Prenatal p25-activated Cdk5 induces pituitary tumorigenesis through MCM2 phosphorylation-mediated cell proliferation
IF 4.8
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
Abstract
Aberrant expression of cyclin-dependent kinase 5 (Cdk5) has been reported in pituitary adenomas. However, the role of Cdk5 in the tumorigenesis remains unclear. We show that prenatal p25-activated Cdk5 phosphorylates minichromosome maintenance protein 2 (Mcm2), enhancing minichromosome maintenance (MCM) family proteins and driving intermediate lobe-located melanotrope-originated pituitary tumorigenesis. In a mouse model with CaMKII promoter-driven transgenic induction of p25, we observed intermediate lobe-originated pituitary adenoma producing non-functional proopiomelanocortin (POMC)-derived peptides under persistent p25 overexpression. Single-cell RNA sequencing revealed Mcm2 may play an important role during tumor progression. Subsequently, Mcm2 was identified as a potential phosphorylated substrate of Cdk5, mediating the tumorous proliferation of melanotrope cells. Silencing Cdk5 or Mcm2 suppressed cell proliferation and colony formation in the 293T cell lines. Therefore, our findings provide a new mouse model of intermediate lobe-originated pituitary adenoma induced by p25/Cdk5 and unveil a previously unappreciated role of Cdk5 and Mcm2 in pituitary adenoma tumorigenesis.
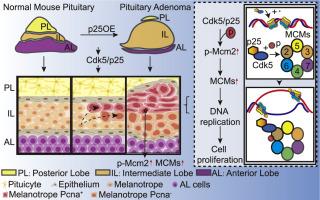
产前 p25 激活的 Cdk5 通过 MCM2 磷酸化介导的细胞增殖诱导垂体肿瘤发生。
据报道,垂体腺瘤中细胞周期蛋白依赖性激酶5(Cdk5)表达异常。然而,Cdk5在肿瘤发生中的作用仍不清楚。我们的研究表明,产前 p25 激活的 Cdk5 可使小染色体维护蛋白 2(Mcm2)磷酸化,从而增强小染色体维护(MCM)家族蛋白的功能,并推动位于中间叶的黑色素细胞起源的垂体瘤的发生。在CaMKII启动子驱动转基因诱导p25的小鼠模型中,我们观察到中间叶起源的垂体腺瘤在p25持续过表达的情况下产生无功能的前黑皮素(POMC)衍生肽。单细胞RNA测序显示,Mcm2可能在肿瘤进展过程中扮演重要角色。随后,Mcm2被确定为Cdk5的潜在磷酸化底物,介导黑素细胞的肿瘤性增殖。沉默Cdk5或Mcm2可抑制293T细胞系的细胞增殖和集落形成。因此,我们的研究结果提供了一种由p25/Cdk5诱导的中间叶源性垂体腺瘤的新小鼠模型,并揭示了Cdk5和Mcm2在垂体腺瘤肿瘤发生中以前未被认识到的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


