Nitrous oxide induces hypothermia and TrkB activation: Maintenance of body temperature abolishes antidepressant-like effects in mice
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Recent studies indicate that nitrous oxide (N2O), a gaseous anesthetic and an NMDA (N-methyl-D-aspartate) receptor antagonist, produces rapid antidepressant effect in patients suffering from treatment-resistant depression. Our recent work implies that hypothermia and reduced energy expenditure are connected with antidepressant-induced activation of TrkB neurotrophin receptors — a key regulator of synaptic plasticity. In this study, we demonstrate that a brief exposure to N2O leads to a drop in body temperature following the treatment, which is linked to decreased locomotor activity; enhanced slow-wave electroencephalographic activity; reduced brain glucose utilization; and increased phosphorylation of TrkB, GSK3β (glycogen synthase kinase 3β), and p70S6K (a kinase downstream of mTor (mammalian target of rapamycin)) in the medial prefrontal cortex of adult male mice. Moreover, preventing the hypothermic response in a chronic corticosterone stress model of depression attenuated the antidepressant-like behavioral effects of N2O in the saccharin preference test. These findings indicate that N2O treatment modulates TrkB signaling and related neurotrophic signaling pathways in a temperature-dependent manner, suggesting that the phenomenon driving TrkB activation — altered thermoregulation and energy expenditure — is linked to antidepressant-like behavioral responses.
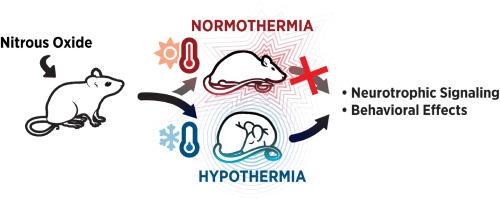
一氧化二氮诱导低体温和 TrkB 激活:维持体温可消除小鼠的抗抑郁样效应
最近的研究表明,一氧化二氮(N2O)是一种气态麻醉剂,也是一种 NMDA(N-甲基-D-天冬氨酸)受体拮抗剂,它能对耐药性抑郁症患者产生快速抗抑郁效果。我们最近的研究表明,低体温和能量消耗减少与抗抑郁药诱导的 TrkB 神经营养素受体激活有关,而 TrkB 神经营养素受体是突触可塑性的关键调节因子。在这项研究中,我们证明了短暂暴露于一氧化二氮会导致治疗后体温下降,而体温下降与运动活动减少、慢波脑电图活动增强、脑葡萄糖利用率降低以及成年雄性小鼠内侧前额叶皮层中TrkB、GSK3β(糖原合酶激酶3β)和p70S6K(mTor(哺乳动物雷帕霉素靶标)下游激酶)的磷酸化增加有关。此外,在慢性皮质酮应激抑郁模型中,防止低体温反应可减轻 N2O 在糖精偏好试验中的抗抑郁样行为效应。这些研究结果表明,氧化亚氮处理以温度依赖的方式调节TrkB信号和相关神经营养信号通路,表明驱动TrkB激活的现象--体温调节和能量消耗的改变--与抗抑郁样行为反应有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


