The thienopyridine A-769662 and benzimidazole 991 inhibit human TASK-3 potassium channels in an AMPK-independent manner
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Heteromeric Tandem pore domain Acid Sensitive (TASK)-1/3 channels are critical to oxygen-sensing by carotid body type 1 cells, where hypoxia-induced inhibition of TASK-3 and/or TASK-1/3 potassium currents leads to voltage-gated calcium entry, exocytotic transmitter release and increases in carotid body afferent input responses that initiate corrective changes in breathing patterns. It was proposed that, in response to hypoxia, the AMP–activated protein kinase (AMPK) might directly phosphorylate and inhibit TASK channels, in particular TASK–3, but studies on rat type I cells questioned this view. However, sequence alignment identified a putative AMPK recognition motif in human (h) TASK-3, but not hTASK–1, with Ser55 representing a potential phosphorylation site. We therefore studied the effects of five different AMPK activators on recombinant hTASK–3 potassium channels expressed in human embryonic kidney (HEK)–293 cells. Two structurally unrelated AMPK activators, the thienopyridine A–769662 (100–500 µM) and the benzimidazole 991 (3–30 µM) inhibited hTASK–3 currents in a concentration–dependent manner, while the 4-azabenzimidazole MK–8722 (3–30 µM) partially inhibited hTASK–3 at concentrations above those required for maximal AMPK activation. By contrast, the 4-azabenzimidazole, BI-9774 (10–100 µM; a closely related analogue of MK8722) and the pro-drug AICA-riboside (1 mM; metabolised to ZMP, an AMP-mimetic) had no significant effect on hTASK–3 currents at concentrations sufficient to maximally activate AMPK. Importantly, A–769662 (300 µM) also inhibited hTASK–3 channel currents in HEK–293 cells that stably over-expressed an AMPK–β1 subunit mutant (S108A) that renders AMPK insensitive to activators that bind to the Allosteric Drug and Metabolite site, such as A–769662. We therefore identify A–769662 and 991 as novel hTASK–3 channel inhibitors and provide conclusive evidence that AMPK does not regulate hTASK–3 channel currents.
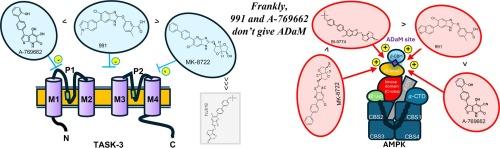
噻吩并吡啶 A-769662 和苯并咪唑 991 以不依赖于 AMPK 的方式抑制人类 TASK-3 钾通道。
异构串联孔域酸敏感(TASK)-1/3 通道对颈动脉体 1 型细胞的氧传感至关重要,缺氧诱导的 TASK-3 和/或 TASK-1/3 钾电流抑制会导致电压门控钙离子进入、外渗递质释放以及颈动脉体传入输入反应的增加,从而引发呼吸模式的纠正变化。有人提出,在应对缺氧时,AMP 激活蛋白激酶(AMPK)可能会直接磷酸化并抑制 TASK 通道,尤其是 TASK-3,但对大鼠 I 型细胞的研究对这一观点提出了质疑。然而,序列比对在人类(h)TASK-3(而非 hTASK-1)中发现了一个潜在的 AMPK 识别基序,其中 Ser55 是一个潜在的磷酸化位点。因此,我们研究了五种不同的 AMPK 激活剂对在人胚胎肾(HEK)-293 细胞中表达的重组 hTASK-3 钾通道的影响。两种结构不相关的 AMPK 激活剂--噻吩并吡啶 A-769662 (100-500 µM)和苯并咪唑 991(3-30 µM)以浓度依赖的方式抑制了 hTASK-3 电流,而 4-氮杂苯并咪唑 MK-8722(3-30 µM)在超过最大 AMPK 激活所需的浓度时部分抑制了 hTASK-3。相比之下,4-氮杂苯并咪唑 BI-9774(10-100 µM;MK8722 的近亲类似物)和原药 AICA 核苷(1 mM;代谢为 ZMP,一种 AMP 模拟物)在足以最大程度激活 AMPK 的浓度下对 hTASK-3 电流没有显著影响。重要的是,A-769662(300 µM)还抑制了稳定过表达 AMPK-β1 亚基突变体(S108A)的 HEK-293 细胞中的 hTASK-3 通道电流,这种突变体使 AMPK 对与异位药物和代谢物位点结合的激活剂(如 A-769662)不敏感。因此,我们确定 A-769662 和 991 为新型 hTASK-3 通道抑制剂,并提供了确凿证据,证明 AMPK 并不调节 hTASK-3 通道电流。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


