Modified (2′-deoxy)adenosines activate autophagy primarily through AMPK/ULK1-dependent pathway
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Autophagy is a conserved self-digestion process, which governs regulated degradation of cellular components. Autophagy is upregulated upon energy shortage sensed by AMP-dependent protein kinase (AMPK). Autophagy activators might be contemplated as therapies for metabolic neurodegenerative diseases and obesity, as well as cancer, considering tumor-suppressive functions of autophagy. Among them, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAr), a nucleoside precursor of the active phosphorylated AMP analog, is the most commonly used pharmacological modulator of AMPK activity, despite its multiple reported “off-target” effects. Here, we assessed the autophagy/mitophagy activation ability of a small set of (2′-deoxy)adenosine derivatives and analogs using a fluorescent reporter assay and immunoblotting analysis. The first two leader compounds, 7,8-dihydro-8-oxo-2′-deoxyadenosine and -adenosine, are nucleoside forms of major oxidative DNA and RNA lesions. The third, a derivative of inactive N6-methyladenosine with a metabolizable phosphate-masking group, exhibited the highest activity in the series. These compounds primarily contributed to the activation of AMPK and outperformed AICAr; however, retaining the activity in knockout cell lines for AMPK (ΔAMPK) and its upstream regulator SIRT1 (ΔSIRT1) suggests that AMPK is not a main cellular target. Overall, we confirmed the prospects of searching for autophagy activators among (2′-deoxy)adenosine derivatives and demonstrated the applicability of the phosphate-masking strategy for increasing their efficacy.
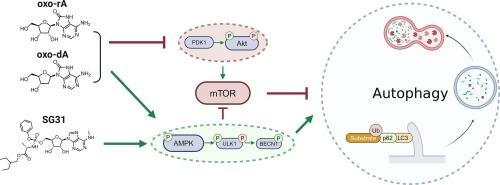
修饰的(2'-脱氧)腺苷主要通过 AMPK/ULK1 依赖性途径激活自噬。
自噬是一种保守的自我消化过程,对细胞成分的降解进行调节。当 AMP 依赖性蛋白激酶(AMPK)感应到能量不足时,自噬功能就会被上调。考虑到自噬的肿瘤抑制功能,自噬激活剂可被视为治疗代谢性神经退行性疾病、肥胖症以及癌症的药物。其中,5-氨基咪唑-4-甲酰胺核糖核苷(AICAr)是一种活性磷酸化 AMP 类似物的核苷前体,是最常用的 AMPK 活性药理调节剂,尽管它有多种 "脱靶 "效应的报道。在这里,我们使用荧光报告分析法和免疫印迹分析法评估了一小部分(2'-脱氧)腺苷衍生物和类似物的自噬/介噬激活能力。前两种领头化合物--7,8-二氢-8-氧代-2'-脱氧腺苷和-腺苷--是主要氧化 DNA 和 RNA 病变的核苷形式。第三种是无活性的 N6-甲基腺苷的衍生物,带有可代谢的磷酸掩蔽基团,在该系列中表现出最高的活性。这些化合物主要促进了 AMPK 的活化,表现优于 AICAr;但是,在 AMPK(ΔAMPK)及其上游调节因子 SIRT1(ΔSIRT1)的基因敲除细胞系中保持活性表明,AMPK 并不是主要的细胞靶标。总之,我们证实了在(2'-脱氧)腺苷衍生物中寻找自噬激活剂的前景,并证明了磷酸盐掩蔽策略对提高其功效的适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


