Systematic review and meta-analysis of preclinical studies
IF 56
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
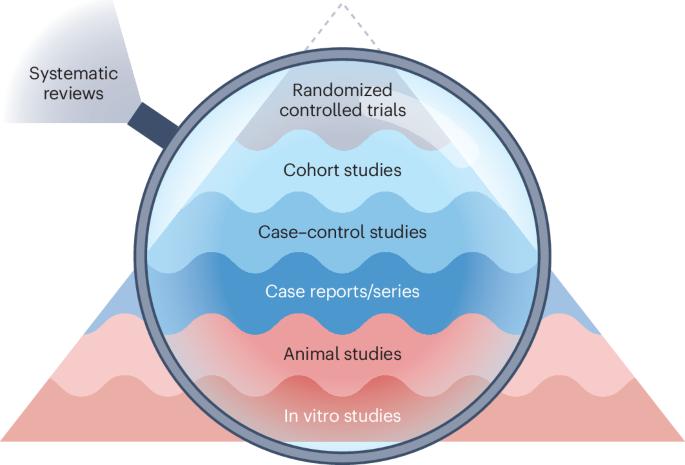
The preclinical research community faces an ever-expanding corpus of biomedical literature, making it challenging to keep abreast with the latest findings. This hampers evidence-based research and informed decision-making. Thus, reliable tools are warranted to manage this evidence and maximize the global investment in research. Systematic reviews, syntheses of existing scientific evidence that address a focused question in an unbiased manner and using explicit methods, have gained momentum as an effective solution. Systematic reviews have an important role in uncovering problems in preclinical research, informing best practice guidelines, reducing research waste, promoting reproducibility and guiding translational research. Systematic reviews of preclinical studies also promote ethical animal use by maximizing the use of existing animal studies, thereby fostering animal welfare. However, poorly performed systematic reviews can produce unreliable results, leading to incorrect conclusions about the underlying literature. This Primer presents guidance for conducting a rigorous systematic review with or without meta-analysis of preclinical studies including animal and in vitro studies. It also discusses the limitations of systematic reviews and outlines current developments such as systematic review automation. By following this Primer, researchers can ensure the rigour and usefulness of their systematic reviews, ultimately benefiting decision-making and research outcomes in preclinical research. Preclinical systematic reviews look at scientific evidence addressing focused questions from animal research studies to inform future clinical research. In this Primer, Ineichen et al. discuss the best practices for conducting preclinical systematic reviews, promoting reproducibility and guiding translational research.
临床前研究的系统回顾和荟萃分析
临床前研究界面临着不断扩大的生物医学文献库,要跟上最新的研究成果具有挑战性。这阻碍了循证研究和知情决策。因此,我们需要可靠的工具来管理这些证据,并最大限度地利用全球的研究投资。系统综述是对现有科学证据的综合,它采用明确的方法,以不偏不倚的方式解决一个重点问题,作为一种有效的解决方案,已经获得了越来越多的关注。系统综述在发现临床前研究中的问题、提供最佳实践指南、减少研究浪费、促进可重复性和指导转化研究方面发挥着重要作用。临床前研究的系统综述还能最大限度地利用现有的动物研究,从而促进动物福利,促进动物使用的道德性。然而,如果系统性综述做得不好,可能会产生不可靠的结果,导致对基础文献得出不正确的结论。本《入门指南》提供了对临床前研究(包括动物和体外研究)进行严格系统综述(无论是否进行荟萃分析)的指导。它还讨论了系统综述的局限性,并概述了系统综述自动化等当前的发展情况。遵循本《入门指南》,研究人员可以确保其系统综述的严谨性和实用性,最终有利于临床前研究的决策和研究成果。临床前系统综述研究科学证据,解决动物研究中的重点问题,为未来的临床研究提供依据。在本《入门》中,Ineichen 等人讨论了进行临床前系统综述、促进可重复性和指导转化研究的最佳实践。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


