N-linked glycosylation affects catalytic parameters and fluctuation of the active center of Aspergillus awamori exo-inulinase
IF 1.4
4区 生物学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Heterogeneous expression of enzymes allows large-scale production with reduced costs. Changes in glycosylation often occur due to changes in the expression host. In the study, the catalytic and biochemical properties of Aspergillus awamori exo-inulinase 1 are compared for A. awamori and Penicillium verruculosum expression hosts. The tertiary structure contains seven sites of N-glycosylation, with two of them located near the active center. If expressed in P. verruculosum, the enzyme was four times less glycosylated and two times more active toward sucrose, raffinose, and stachyose due to an increase in kcat. These substrates with a short chain of 2–4 monosaccharide units were used to characterize the interaction of the substrate with the amino acid residues in the active center while preventing the interaction of the substrate with N-linked glycans. Molecular dynamics simulations showed an increase in the fluctuation of the active center with an increase in the length of N-linked glycans. The fluctuation of the residues N40 and Q57, which interact with the hydroxyl group O5 of the fructose unit in the −1 subsite of the active center, was increased by 1.6 times. The fluctuation of the residue W335, which interacts with the hydroxyl group O1 of the fructose unit together with the catalytic residue D41 and affects the torsion angle geometry of the substrate molecules, was increased by 1.5 times. The residue R188, which analogously to W335 affects the torsion angle geometry of the substrate molecules, was also among the affected residues with a 1.2-fold increase in the fluctuation.
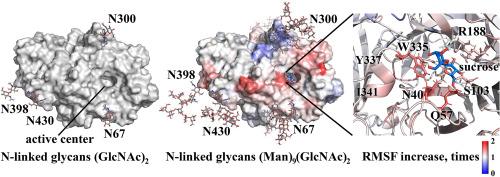
N-连接的糖基化影响黑曲霉 awamori 外胰岛素酶活性中心的催化参数和波动。
酶的异构表达可以大规模生产,降低成本。糖基化的变化往往是由表达宿主的变化引起的。在这项研究中,比较了阿瓦莫里曲霉外胰岛素酶 1 在阿瓦莫里曲霉和疣青霉表达宿主下的催化和生化特性。其三级结构包含七个 N-糖基化位点,其中两个位于活性中心附近。如果在疣青霉中表达,由于 kcat 的增加,该酶的糖基化程度降低四倍,对蔗糖、棉子糖和水苏糖的活性提高两倍。这些底物具有 2-4 个单糖单位的短链,用于表征底物与活性中心氨基酸残基的相互作用,同时防止底物与连接糖的相互作用。分子动力学模拟显示,活性中心的波动会随着 N 联糖类长度的增加而增加。残基 N40 和 Q57 与活性中心 -1 亚位点果糖单元的羟基 O5 相互作用,其波动增加了 1.6 倍。与催化残基 D41 一起与果糖单元的羟基 O1 相互作用并影响底物分子扭转角几何形状的残基 W335 的波动增加了 1.5 倍。与 W335 类似影响底物分子扭转角几何形状的残基 R188 也是受影响的残基之一,其波动增加了 1.2 倍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Protein expression and purification
生物-生化研究方法
CiteScore
3.70
自引率
6.20%
发文量
120
审稿时长
32 days
期刊介绍:
Protein Expression and Purification is an international journal providing a forum for the dissemination of new information on protein expression, extraction, purification, characterization, and/or applications using conventional biochemical and/or modern molecular biological approaches and methods, which are of broad interest to the field. The journal does not typically publish repetitive examples of protein expression and purification involving standard, well-established, methods. However, exceptions might include studies on important and/or difficult to express and/or purify proteins and/or studies that include extensive protein characterization, which provide new, previously unpublished information.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


