Comparison of DNA methylation based classification models for precision diagnostics of central nervous system tumors
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
As part of the advancement in therapeutic decision-making for brain tumor patients at St. Jude Children’s Research Hospital (SJCRH), we developed three robust classifiers, a deep learning neural network (NN), k-nearest neighbor (kNN), and random forest (RF), trained on a reference series DNA-methylation profiles to classify central nervous system (CNS) tumor types. The models’ performance was rigorously validated against 2054 samples from two independent cohorts. In addition to classic metrics of model performance, we compared the robustness of the three models to reduced tumor purity, a critical consideration in the clinical utility of such classifiers. Our findings revealed that the NN model exhibited the highest accuracy and maintained a balance between precision and recall. The NN model was the most resistant to drops in performance associated with a reduction in tumor purity, showing good performance until the purity fell below 50%. Through rigorous validation, our study emphasizes the potential of DNA-methylation-based deep learning methods to improve precision medicine for brain tumor classification in the clinical setting.
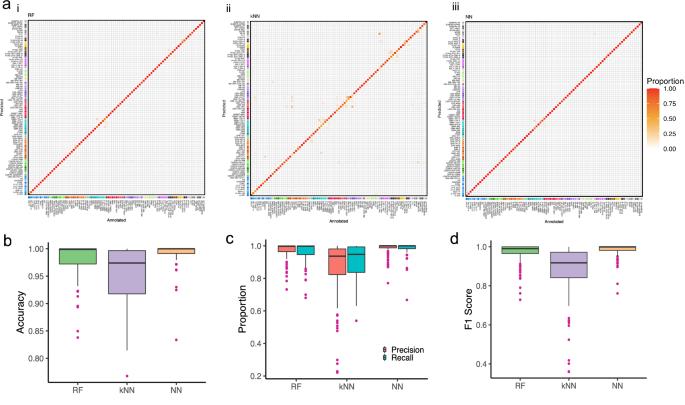
基于 DNA 甲基化分类模型的中枢神经系统肿瘤精确诊断比较。
作为圣裘德儿童研究医院(SJCRH)脑肿瘤患者治疗决策研究进展的一部分,我们开发了三种稳健的分类器,即深度学习神经网络(NN)、k-近邻(kNN)和随机森林(RF),它们都是根据参考系列 DNA 甲基化图谱训练而成,用于对中枢神经系统(CNS)肿瘤类型进行分类。这些模型的性能通过来自两个独立队列的 2054 个样本进行了严格验证。除了经典的模型性能指标外,我们还比较了三种模型对肿瘤纯度降低的稳健性,这也是此类分类器在临床应用中的一个重要考虑因素。我们的研究结果表明,NN 模型的准确率最高,并在精确度和召回率之间保持了平衡。NN 模型对肿瘤纯度降低带来的性能下降的抵抗力最强,在纯度低于 50%之前一直表现良好。通过严格的验证,我们的研究强调了基于 DNA 甲基化的深度学习方法在提高临床脑肿瘤分类精准医疗方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


