Genetic deletion of the apoptosis associated speck like protein containing a card in LysM+ macrophages attenuates spinal cord injury by regulating M1/M2 polarization through ASC-dependent inflammasome signaling axis
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Apoptosis associated speck like protein containing a card (ASC), the key adaptor protein of the assembly and activation of canonical inflammasomes, has been found to play a significant role in neuroinflammation after spinal cord injury (SCI). The previous studies indicated that widely block or knockout ASC can ameliorate SCI. However, ASC is ubiquitously expressed in infiltrated macrophages and local microglia, so further exploration is needed on which type of cell playing the key role. In this study, using the LysMcre;Ascflox/flox mice with macrophage-specifc ASC conditional knockout (CKO) and contusive SCI model, we focus on evaluating the specific role of ASC in lysozyme 2 (LysM)+ myeloid cells (mainly infiltrated macrophages) in this pathology. The results revealed that macrophage-specifc Asc CKO exhibited the follow effects: (1) A significant reduction in the numbers of infiltrated macrophages in the all phases of SCI, and activated microglia in the acute and subacute phases. (2) A significant reduction in ASC, caspase-1, interleukin (IL)-1β, and IL-18 compared to control mice. (3) In the acute and subacute phases of SCI, M1 subset differentiation was inhibited, and M2 differentiation was increased. (4) Histology and hindlimb motor recoveries were improved. In conclusion, this study elucidates that macrophage-specific ASC CKO can improve nerve function recovery after SCI by regulating M1/M2 polarization through inhibiting ASC-dependent inflammasome signaling axis. This indicates that ASC in peripheral infiltrated macrophages may play an important role in SCI pathology, at least in mice, could be a potential target for treatment.
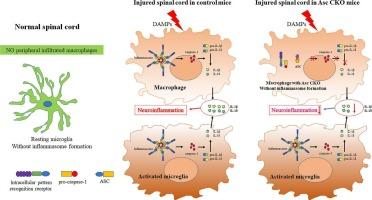
在 LysM+ 巨噬细胞中遗传性缺失含有卡片的凋亡相关斑点样蛋白,可通过 ASC 依赖性炎症小体信号轴调节 M1/M2 极化,从而减轻脊髓损伤。
研究发现,凋亡相关的含卡斑点样蛋白(ASC)是组装和激活典型炎性体的关键适配蛋白,在脊髓损伤(SCI)后的神经炎症中发挥着重要作用。之前的研究表明,广泛阻断或敲除 ASC 可以改善 SCI。然而,ASC 在浸润的巨噬细胞和局部小胶质细胞中普遍表达,因此需要进一步研究哪种细胞起关键作用。在本研究中,我们利用巨噬细胞特异性 ASC 条件性敲除(CKO)的 LysMcre;Ascflox/flox 小鼠和挫伤性 SCI 模型,重点评估了 ASC 在溶菌酶 2(LysM)+ 髓系细胞(主要是浸润的巨噬细胞)在这一病理过程中的特异性作用。结果发现,巨噬细胞特异性 Asc CKO 表现出以下效应:(1)在 SCI 的所有阶段,浸润的巨噬细胞数量显著减少,在急性和亚急性阶段,激活的小胶质细胞数量显著减少。(2)与对照组小鼠相比,ASC、caspase-1、白细胞介素(IL)-1β 和 IL-18 明显减少。(3)在 SCI 急性期和亚急性期,M1 亚群分化受到抑制,M2 亚群分化增加。(4)组织学和后肢运动恢复得到改善。总之,本研究阐明了巨噬细胞特异性 ASC CKO 可通过抑制 ASC 依赖的炎性体信号轴调节 M1/M2 极化,从而改善 SCI 后的神经功能恢复。这表明外周浸润巨噬细胞中的ASC可能在SCI病理中扮演重要角色,至少在小鼠中是如此,并可能成为潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


