Filament formation activates protease and ring nuclease activities of CRISPR Lon-SAVED
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
To combat phage infection, type III CRISPR-Cas systems utilize cyclic oligoadenylates (cAn) signaling to activate various auxiliary effectors, including the CRISPR-associated Lon-SAVED protease CalpL, which forms a tripartite effector system together with an anti-σ factor, CalpT, and an ECF-like σ factor, CalpS. Here, we report the characterization of the Candidatus Cloacimonas acidaminovorans CalpL-CalpT-CalpS. We demonstrate that cA4 binding triggers CalpL filament formation and activates it to cleave CalpT within the CalpT-CalpS dimer. This cleavage exposes the CalpT C-degron, which targets it for further degradation by cellular proteases. Consequently, CalpS is released to bind to RNA polymerase, causing growth arrest in E. coli. Furthermore, the CalpL-CalpT-CalpS system is regulated by the SAVED domain of CalpL, which is a ring nuclease that cleaves cA4 in a sequential three-step mechanism. These findings provide key mechanistic details for the activation, proteolytic events, and regulation of the signaling cascade in the type III CRISPR-Cas immunity.
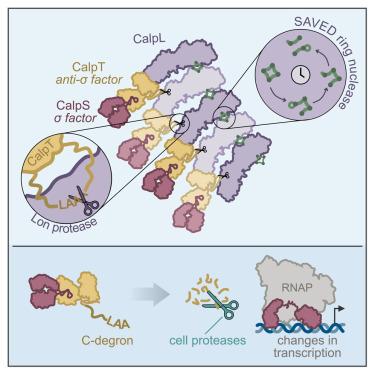
丝状体的形成激活了 CRISPR Lon-SAVED 的蛋白酶和环状核酸酶活性
为了对抗噬菌体感染,III型CRISPR-Cas系统利用环状寡腺苷酸(cAn)信号来激活各种辅助效应器,包括CRISPR相关的Lon-SAVED蛋白酶CalpL,它与抗σ因子CalpT和类ECFσ因子CalpS一起构成了一个三方效应器系统。在这里,我们报告了 Cloacimonas acidaminovorans 杆菌 CalpL-CalpT-CalpS 的特征。我们证明,cA4 的结合会触发 CalpL 长丝的形成,并激活它裂解 CalpT-CalpS 二聚体中的 CalpT。这种裂解暴露了 CalpT 的 C-半导,使其成为细胞蛋白酶进一步降解的目标。因此,CalpS 被释放出来与 RNA 聚合酶结合,导致大肠杆菌生长停滞。此外,CalpL-CalpT-CalpS 系统还受 CalpL 的 SAVED 结构域调控,SAVED 结构域是一种环状核酸酶,能以三步顺序机制裂解 cA4。这些发现为III型CRISPR-Cas免疫中信号级联的激活、蛋白水解事件和调控提供了关键的机制细节。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


