Cellular taxonomy of the preleukemic bone marrow niche of acute myeloid leukemia
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
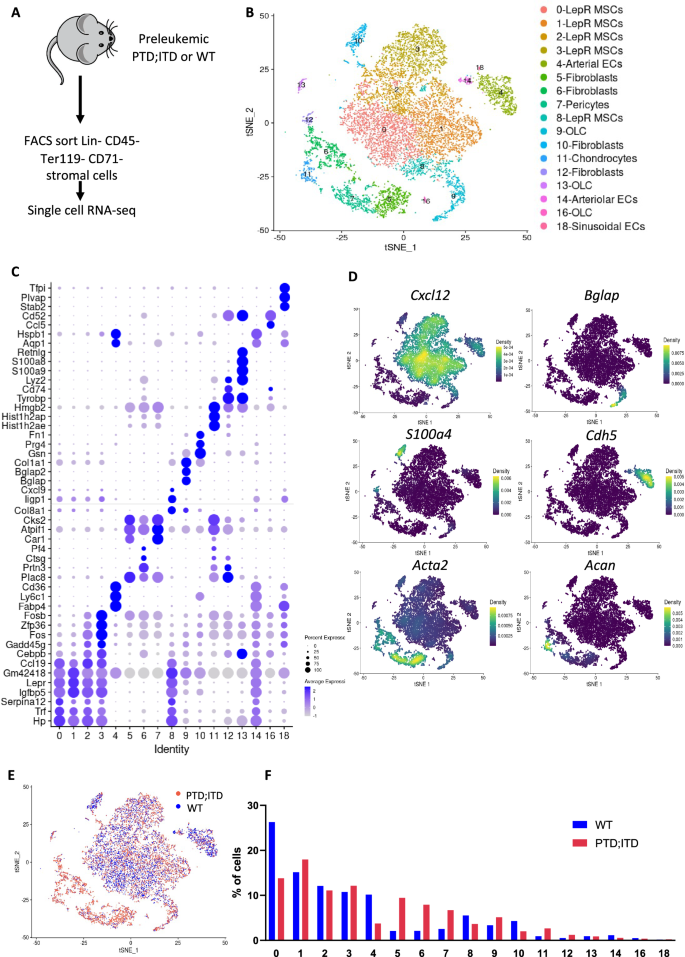
Leukemias arise from recurrent clonal mutations in hematopoietic stem/progenitor cells (HSPCs) that cause profound changes in the bone marrow microenvironment (BMM) favoring leukemic stem cell (LSC) growth over normal HSPCs. Understanding the cross talk between preleukemic mutated HSPCs and the BMM is critical to develop novel therapeutic strategies to prevent leukemogenesis. We hypothesize that preleukemic-LSCs (pLSCs) induce BMM changes critical for leukemogenesis. Using our AML-murine model, we performed single-cell RNA-sequencing of preleukemic BMM (pBMM) cells. We found normal HSC (nHSC)-regulating LepR+ mesenchymal stem cells, and endothelial cells were decreased, along with increases in CD55+ fibroblasts and pericytes. Preleukemic CD55+ fibroblasts had higher proliferation rates and decreased collagen expression, suggesting extracellular matrix remodeling during leukemogenesis. Importantly, co-culture assays found preleukemic CD55+ fibroblasts expanded pLSCs significantly over nHSCs. In conclusion, we have identified a distinct pBMM and a novel CD55+ fibroblast population that is expanded in pBMM that promote fitness of pLSCs over nHSCs.

急性髓性白血病白血病前骨髓生态位的细胞分类法
白血病源于造血干细胞/祖细胞(HSPCs)的反复克隆突变,这种突变导致骨髓微环境(BMM)发生深刻变化,有利于白血病干细胞(LSC)的生长,而不是正常的HSPCs。了解白血病变异前HSPC与骨髓微环境之间的交叉对话,对于开发预防白血病发生的新型治疗策略至关重要。我们假设白血病前造血干细胞(pLSCs)会诱导对白血病发生至关重要的BMM变化。我们利用急性髓细胞性白血病-鼠模型,对白血病前期 BMM(pBMM)细胞进行了单细胞 RNA 测序。我们发现正常造血干细胞(nHSC)调节的LepR+间充质干细胞和内皮细胞减少,CD55+成纤维细胞和周细胞增加。白血病前CD55+成纤维细胞的增殖率更高,胶原表达减少,这表明细胞外基质在白血病发生过程中发生了重塑。重要的是,共培养试验发现,白血病前期 CD55+ 成纤维细胞对 pLSCs 的扩增明显高于 nHSCs。总之,我们发现了一种独特的 pBMM 和一种新的 CD55+ 成纤维细胞群,它们在 pBMM 中扩增,促进了 pLSCs 对 nHSCs 的适应性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


