The epigenetic state of the cell of origin defines mechanisms of leukemogenesis
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Acute myeloid leukemia (AML) shows variable clinical outcome. The normal hematopoietic cell of origin impacts the clinical behavior of AML, with AML from hematopoietic stem cells (HSCs) prone to chemotherapy resistance in model systems. However, the mechanisms by which HSC programs are transmitted to AML are not known. Here, we introduce the leukemogenic MLL-AF9 translocation into defined human hematopoietic populations, finding that AML from HSCs is enriched for leukemic stem cells (LSCs) compared to AML from progenitors. By epigenetic profiling, we identify a putative inherited program from the normal HSC that collaborates with oncogene-driven programs to confer aggressive behavior in HSC-AML. We find that components of this program are required for HSC-AML growth and survival and identify RNA polymerase (RNAP) II-mediated transcription as a therapeutic vulnerability. Overall, we propose a mechanism as to how epigenetic programs from the leukemic cell of origin are inherited through transformation to impart the clinical heterogeneity of AML.
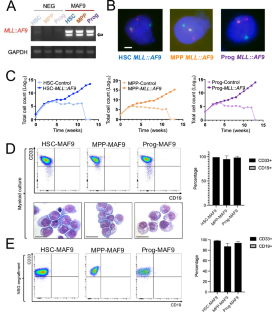

起源细胞的表观遗传状态决定了白血病的发生机制。
急性髓性白血病(AML)的临床结果各不相同。正常造血细胞的来源会影响急性髓性白血病的临床表现,在模型系统中,来自造血干细胞(HSC)的急性髓性白血病容易产生化疗耐药性。然而,造血干细胞程序传递给急性髓细胞性白血病的机制尚不清楚。在这里,我们把致白血病的MLL-AF9易位引入到确定的人类造血群体中,发现与来自祖细胞的急性髓细胞性白血病相比,来自造血干细胞的急性髓细胞性白血病富含白血病干细胞(LSC)。通过表观遗传学分析,我们确定了一种来自正常造血干细胞的假定遗传程序,该程序与癌基因驱动的程序合作,赋予造血干细胞-急性髓细胞性白血病以侵袭行为。我们发现该程序的组成部分是 HSC-AML 生长和存活所必需的,并确定 RNA 聚合酶 (RNAP) II 介导的转录是一种治疗漏洞。总之,我们提出了一种机制,说明白血病原代细胞的表观遗传学程序是如何通过转化遗传给急性髓细胞性白血病的临床异质性的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


