Paired Electrocatalysis-Enabled Cross Coupling of Sulfinamides with Olefins toward the Synthesis of Vinyl Sulfoximines.
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We present here a novel paired electrocatalysis-enabled convenient synthesis of the (E)-vinyl sulfoximines through the cross-coupling reaction of sulfinamides and olefins. This protocol showed a broad substrate scope and excellent E selectivity of products under metal- and oxidant-free conditions. A preliminary mechanistic study suggested that fluorinated sulfoximine generated from anodic oxidation of sulfinamide was the key intermediate that was then converted into the sulfonimidoyl radical at the cathode with the help of DBU in this reaction.
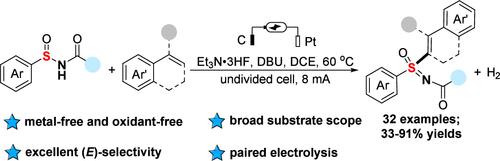
利用成对电催化将亚磺酰胺与烯烃进行交叉偶联以合成乙烯基亚磺酰亚胺。
我们在此介绍一种新型成对电催化技术,通过亚磺酰胺与烯烃的交叉偶联反应,方便地合成(E)-乙烯基亚磺酰亚胺。在无金属和氧化剂的条件下,该方法显示出广泛的底物范围和优异的产品选择性。初步的机理研究表明,亚磺酰胺阳极氧化生成的氟化亚磺酰亚胺是该反应的关键中间体,然后在阴极上借助 DBU 转化为磺酰亚胺基。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


