Generating globin-like reactivities in [human serum albumin-FeII(heme)] complex through N-donor ligand addition
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
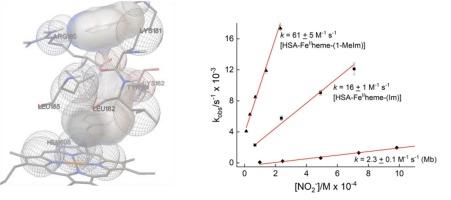
Human serum albumin (HSA) has a strong binding affinity for heme b, forming a complex in a 1:1 ratio with the co-factor ([HSA-FeIIIheme]). This system displays spectroscopic and functional properties comparable to globins when chemical derivatives mimicking them are incorporated into the protein matrix. The aim of this study is to generate globin-like systems using [HSA-FeIIIheme] as a protein template and binding N-donor ligands (imidazole, Im; and 1-methylimidazole, 1-MeIm) to construct artificial [HSA-Fe(heme)-(N-donor)] complexes. Their electronic structure and binding thermodynamics are investigated using UV–vis and (synchronous) fluorescence spectroscopies, while ligand-protein interactions are visualized using docking simulations. The imidazole derivatives have a strong affinity for [HSA-FeIIIheme] (K ∼ 104–106), where the spontaneous binding of Im and 1-MeIm are dominated by entropic and enthalpic effects, respectively. The reduced form of the [HSA-Fe(heme)-(N-donor)] complexes demonstrate nitrite reductase (NiR) activity similar to that observed in globins, but with significant differences in their rates. [HSA-FeIIheme-(1-MeIm)] reduces nitrite ∼4× faster than the Im analogue, and ∼ 30× faster than myoglobin (Mb). The enhanced NiR activity of [HSA-FeIIheme-(1-MeIm)] is a cumulative effect of several factors including a slightly expanded and more optimal heme binding pocket, nearby residues as possible proton sources, and a H-bonding interaction between 1-MeIm and residues Arg160 and Lys181 that may have a long-distance influence on the heme π electron density.
通过添加 N-供体配体在[人血清白蛋白-FeII(血红素)]复合物中产生类似球蛋白的反应性
人血清白蛋白(HSA)与血红素 b 有很强的结合亲和力,能与辅助因子([HSA-FeIIIheme])以 1:1 的比例形成复合物。当模仿球蛋白的化学衍生物加入到蛋白质基质中时,该系统显示出与球蛋白相当的光谱和功能特性。本研究的目的是利用[HSA-FeIIIheme]作为蛋白质模板,并结合 N-供体配体(咪唑,Im;和 1-甲基咪唑,1-MeIm)来构建人工[HSA-Fe(heme)-(N-供体)]复合物,从而生成类似球蛋白的系统。利用紫外-可见光谱和(同步)荧光光谱研究了它们的电子结构和结合热力学,同时利用对接模拟将配体与蛋白质的相互作用可视化。咪唑衍生物与[HSA-FeIIIheme]具有很强的亲和力(K ∼ 104-106),其中 Im 和 1-MeIm 的自发结合分别受熵效应和焓效应的支配。还原形式的[HSA-Fe(血红素)-(N-供体)]复合物显示出与在球蛋白中观察到的类似的亚硝酸盐还原酶(NiR)活性,但它们的还原速率有显著差异。[HSA-FeIIheme-(1-MeIm)]还原亚硝酸盐的速度比 Im 类似物快 4 倍,比肌红蛋白(Mb)快 30 倍。HSA-FeIIheme-(1-MeIm)]的 NiR 活性增强是多种因素的累积效应,包括血红素结合口袋略有扩大且更为理想,附近的残基可能是质子源,以及 1-MeIm 与 Arg160 和 Lys181 残基之间的 H 键相互作用可能对血红素 π 电子密度产生长距离影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


