Design, synthesis and biological various aryl derivatives of (pyridin-4-yl) imidazo[1,5-a]pyridin-1-yl)oxazoles as anticancer agents
IF 2.7
Q2 Chemistry
引用次数: 0
Abstract
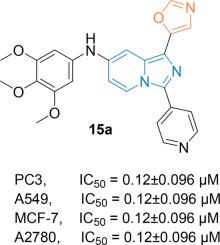
A new library of various aryl derivatives of (pyridin-4-yl)imidazo[1,5-a]pyridin-1-yl)oxazoles (10a-j) and their chemical structures were confirmed by analytical data. Further, the newly derived aryl derivatives (10a-j) were evaluated for their preliminary anticancer applications towards four human cancer cell lines, such as human prostate cancer (PC3), human lung cancer (A549), human breast cancer (MCF-7) & human ovarian cancer (A2780) by employing the MTT method. Most of the evaluated compounds displayed remarkable activity as compared with the standard reference, etoposide. The results found that these compounds (10a, 10b, 10c, 10d and 10e) showed more potent activity than standard. Among them, the compound 10a with 3,4,5-trimethoxy electron donating substituent on the aryl skeleton exhibited most promising activity (PC3 = 0.12±0.096 µM; A549=0.43±0.087 µM; MCF-7 = 0.21±0.093 µM & A2780=0.47±0.083 µM.
作为抗癌剂的 (吡啶-4-基) 咪唑并[1,5-a]吡啶-1-基)噁唑的各种芳基衍生物的设计、合成和生物学特性
通过分析数据确认了一个新的(吡啶-4-基)咪唑并[1,5-a]吡啶-1-基)恶唑芳基衍生物库(10a-j)及其化学结构。此外,还采用 MTT 法评估了新衍生的芳基衍生物(10a-j)对四种人类癌细胞系(如人类前列腺癌(PC3)、人类肺癌(A549)、人类乳腺癌(MCF-7)和amp;人类卵巢癌(A2780))的初步抗癌应用。与标准参照物依托泊苷相比,大多数被评估的化合物都显示出显著的活性。结果发现,这些化合物(10a、10b、10c、10d 和 10e)显示出比标准化合物更强的活性。其中,芳基骨架上带有 3,4,5-三甲氧基电子捐赠取代基的化合物 10a 表现出最有希望的活性(PC3=0.12±0.096 µM;A549=0.43±0.087 µM;MCF-7=0.21±0.093 µM & A2780=0.47±0.083 µM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Data Collections
Chemistry-Chemistry (all)
CiteScore
6.10
自引率
0.00%
发文量
169
审稿时长
24 days
期刊介绍:
Chemical Data Collections (CDC) provides a publication outlet for the increasing need to make research material and data easy to share and re-use. Publication of research data with CDC will allow scientists to: -Make their data easy to find and access -Benefit from the fast publication process -Contribute to proper data citation and attribution -Publish their intermediate and null/negative results -Receive recognition for the work that does not fit traditional article format. The research data will be published as ''data articles'' that support fast and easy submission and quick peer-review processes. Data articles introduced by CDC are short self-contained publications about research materials and data. They must provide the scientific context of the described work and contain the following elements: a title, list of authors (plus affiliations), abstract, keywords, graphical abstract, metadata table, main text and at least three references. The journal welcomes submissions focusing on (but not limited to) the following categories of research output: spectral data, syntheses, crystallographic data, computational simulations, molecular dynamics and models, physicochemical data, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


