Characterisation of APOBEC3B-Mediated RNA editing in breast cancer cells reveals regulatory roles of NEAT1 and MALAT1 lncRNAs
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
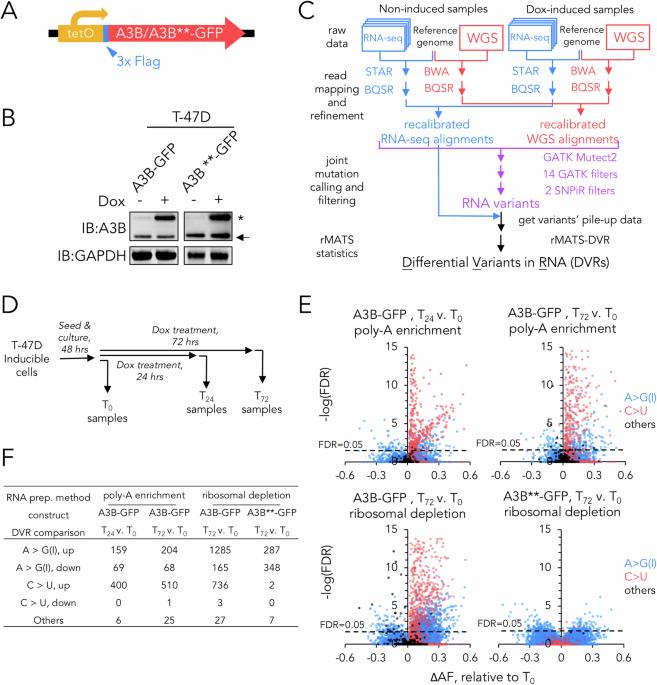
RNA editing is a crucial post-transcriptional process that influences gene expression and increases the diversity of the proteome as a result of amino acid substitution. Recently, the APOBEC3 family has emerged as a significant player in this mechanism, with APOBEC3A (A3A) having prominent roles in base editing during immune and stress responses. APOBEC3B (A3B), another family member, has gained attention for its potential role in generating genomic DNA mutations in breast cancer. In this study, we coupled an inducible expression cell model with a novel methodology for identifying differential variants in RNA (DVRs) to map A3B-mediated RNA editing sites in a breast cancer cell model. Our findings indicate that A3B engages in selective RNA editing including targeting NEAT1 and MALAT1 long non-coding RNAs that are often highly expressed in tumour cells. Notably, the binding of these RNAs sequesters A3B and suppresses global A3B activity against RNA and DNA. Release of A3B from NEAT1/MALAT1 resulted in increased A3B activity at the expense of A3A activity suggesting a regulatory feedback loop between the two family members. This research substantially advances our understanding of A3B’s role in RNA editing, its mechanistic underpinnings, and its potential relevance in the pathogenesis of breast cancer.
乳腺癌细胞中 APOBEC3B 介导的 RNA 编辑特征揭示了 NEAT1 和 MALAT1 lncRNA 的调控作用。
RNA 编辑是一个关键的转录后过程,它能影响基因表达,并通过氨基酸置换增加蛋白质组的多样性。最近,APOBEC3 家族在这一机制中扮演了重要角色,其中 APOBEC3A(A3A)在免疫和应激反应过程中的碱基编辑中发挥了突出作用。另一个家族成员 APOBEC3B(A3B)因其在乳腺癌基因组 DNA 突变中的潜在作用而备受关注。在这项研究中,我们将诱导表达细胞模型与鉴定 RNA 差异变体(DVRs)的新方法相结合,在乳腺癌细胞模型中绘制了 A3B 介导的 RNA 编辑位点图。我们的研究结果表明,A3B 参与了选择性 RNA 编辑,包括靶向 NEAT1 和 MALAT1 长非编码 RNA,这些 RNA 通常在肿瘤细胞中高表达。值得注意的是,这些 RNA 的结合会封存 A3B 并抑制 A3B 针对 RNA 和 DNA 的整体活性。将 A3B 从 NEAT1/MALAT1 中释放后,A3B 的活性增加,而 A3A 的活性降低,这表明这两个家族成员之间存在一个调控反馈回路。这项研究极大地推动了我们对 A3B 在 RNA 编辑中的作用、其机理基础及其在乳腺癌发病机制中的潜在相关性的了解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


