Hypoxic stabilization of RIPOR3 mRNA via METTL3-mediated m6A methylation drives breast cancer progression and metastasis
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Dysregulated N6-methyladenosine (m6A) modification has been associated with breast cancer pathogenesis. Hypoxia which characterizes solid tumors is known to reprogram the m6A epitranscriptome, but the underlying mechanisms of how this process contributes to breast cancer progression remain poorly understood. Through integrative analyses of m6A-RIP sequencing and RNA sequencing databases, we reveal a cluster of mRNAs with upregulated m6A methylation and expression under hypoxia, that are enriched by many oncogenic pathways, including PI3K–Akt signaling. Furthermore, we identify the mRNA, RIPOR3, as a target of METTL3-mediated m6A methylation in response to hypoxia. We find that m6A methylation stabilizes RIPOR3, increasing its protein expression in a METTL3 catalytic activity-dependent manner, and consequently driving breast tumor growth and metastasis. RIPOR3 is found to be overexpressed in breast cancer cell lines and tumor tissues from breast cancer patients, in whom elevated RIPOR3 is associated with a worse prognosis. Mechanistically, we show that RIPOR3 interacts with EGFR and is essential for the PI3K–Akt pathway activation. In conclusion, we identify RIPOR3 as a hypoxia-stabilized oncogenic driver via METTL3-mediated m6A methylation, thus provide a potential therapeutic target for breast cancer.
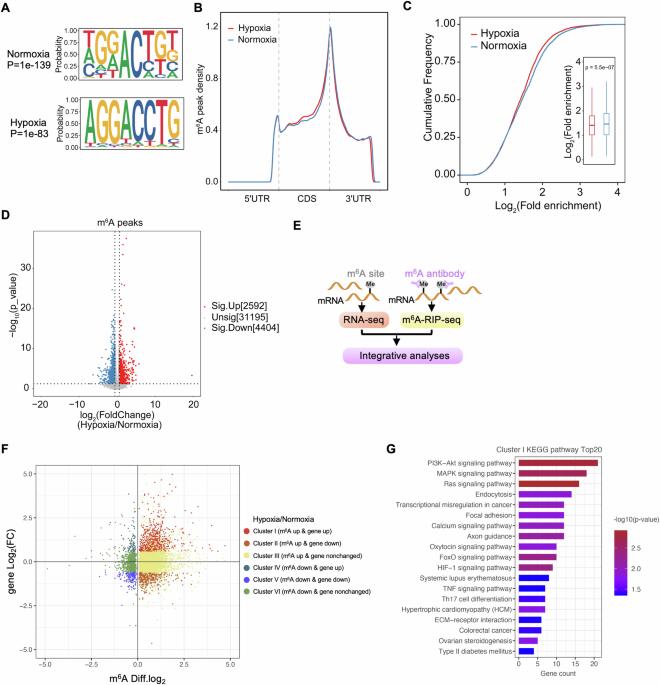
通过 METTL3 介导的 m6A 甲基化使 RIPOR3 mRNA 低氧稳定,从而推动乳腺癌的进展和转移。
N6-甲基腺苷(m6A)修饰失调与乳腺癌的发病机制有关。众所周知,实体瘤所特有的缺氧会对 m6A 表转录组进行重编程,但人们对这一过程如何导致乳腺癌进展的潜在机制仍然知之甚少。通过对 m6A-RIP 测序和 RNA 测序数据库的综合分析,我们揭示了缺氧条件下 m6A 甲基化和表达上调的 mRNA 群,这些 mRNA 在包括 PI3K-Akt 信号转导在内的许多致癌通路中都有富集。此外,我们还发现 RIPOR3 这一 mRNA 是 METTL3 介导的 m6A 甲基化对缺氧反应的靶标。我们发现,m6A 甲基化会稳定 RIPOR3,以 METTL3 催化活性依赖的方式增加其蛋白表达,从而推动乳腺肿瘤的生长和转移。研究发现,RIPOR3 在乳腺癌细胞系和乳腺癌患者的肿瘤组织中过度表达,RIPOR3 的升高与预后恶化有关。从机理上讲,我们发现 RIPOR3 与表皮生长因子受体(EGFR)相互作用,对 PI3K-Akt 通路的激活至关重要。总之,我们通过 METTL3 介导的 m6A 甲基化,发现 RIPOR3 是一种低氧稳定的致癌驱动因子,从而为乳腺癌提供了一个潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


