Reversal of neurodevelopmental impairment and cognitive enhancement by pharmacological intervention with the polyphenol polydatin in a Down syndrome model
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Intellectual disability (ID) is the unavoidable hallmark of Down syndrome (DS), a genetic condition due to triplication of chromosome 21. ID in DS is largely attributable to neurogenesis and dendritogenesis alterations taking place in the prenatal/neonatal period, the most critical time window for brain development. There are currently no treatments for ID in DS. Considering the timeline of brain development, treatment aimed at improving the neurological phenotypes of DS should be initiated as early as possible and use safe agents. The goal of this study was to establish whether it is possible to improve DS-linked neurodevelopmental defects through early treatment with polydatin, a natural polyphenol. We used the Ts65Dn mouse model of DS and focused on the hippocampus, a brain region fundamental for long-term memory. We found that in Ts65Dn mice of both sexes treated with polydatin from postnatal (P) day 3 to P15 there was full restoration of neurogenesis, neuron number, and dendritic development. These effects were accompanied by normalization of Cyclin D1 and DSCAM levels, which may account for the rescue of neurogenesis and dendritogenesis, respectively. Importantly, in Ts65Dn mice treated with polydatin from P3 to adolescence (∼P50) there was full restoration of hippocampus-dependent memory, indicating a pro-cognitive outcome of treatment. No adverse effects were observed on the body and brain weight. The efficacy and safety of polydatin in a model of DS prospect the possibility of its use during early life stages for amelioration of DS-linked neurodevelopmental alterations.
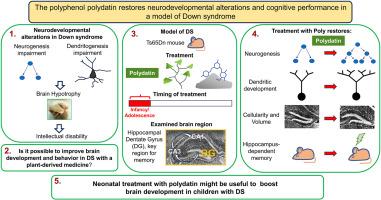
在唐氏综合征模型中使用多酚多苷进行药物干预,可逆转神经发育障碍并增强认知能力。
智力障碍(ID)是唐氏综合征(DS)不可避免的特征,而唐氏综合征是一种因 21 号染色体三倍体而导致的遗传病。唐氏综合征的智力障碍主要是由于产前/新生儿期的神经发生和树突发生改变造成的,而这一时期正是大脑发育最关键的时期。目前尚无治疗 DS 先天性脑发育症的方法。考虑到大脑发育的时间表,旨在改善 DS 神经表型的治疗应尽早开始,并使用安全的药物。本研究的目的是确定是否有可能通过使用天然多酚多拉丁(polydatin)进行早期治疗来改善与DS相关的神经发育缺陷。我们使用了Ts65Dn DS小鼠模型,并重点研究了海马区--一个对长期记忆至关重要的脑区。我们发现,从出生后第 3 天到出生后第 15 天,使用多酚类药物治疗的雌雄 Ts65Dn 小鼠的神经发生、神经元数量和树突发育都得到了完全恢复。伴随这些影响的是细胞周期蛋白 D1 和 DSCAM 水平的正常化,这可能是神经发生和树突形成得到挽救的原因。重要的是,Ts65Dn小鼠在P3至青春期(∼P50)期间接受多铂治疗后,海马依赖性记忆完全恢复,这表明治疗具有促进认知的效果。没有观察到对身体和大脑重量的不良影响。多拉丁在DS模型中的有效性和安全性为在生命早期阶段使用多拉丁来改善与DS相关的神经发育改变提供了可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


