Inhibition of KIF5b-mediated Nav1.8 transport by ropivacaine contributes to axonal regeneration following sciatic nerve injury in rats
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Peripheral nerve injury (PNI), typically caused by traumatic accidents or medical events, is currently one of the most common diseases that leads to limb disability. After PNI, tetrodotoxin-resistant voltage-gated sodium channel Nav1.8 is upregulated at the lesion site. Our earlier study suggested that ropivacaine promotes axon regrowth by regulating Nav1.8-mediated macrophage signaling. Nevertheless, the mechanism of ropivacaine in regulation of Nav1.8 expression remains incompletely understood. Kinesin family 5b (KIF5b) was reported to mediate the Nav1.8 axonal transport from dorsal root ganglia (DRGs) to lesion site. Herein, we investigated whether ropivacaine promotes axon regeneration through inhibition of KIF5b-mediated Nav1.8 transport. Reduced levels of KIF5b and Nav1.8 in DRGs coincide with their increase at the lesion site. Nav1.8 mRNA was significantly increased at the lesion site but not in DRGs. Surprisingly, ropivacaine reversed the alterations of Nav1.8 and KIF5b protein expression without affecting Nav1.8 mRNA level. Due to KIF5b overexpression in DRGs, Nav1.8 protein level was significantly decreased in DRGs and increased at the lesion site. We also found KIF5b overexpression significantly impaired behavioral functions, reduced the recovery index of compound muscle action potential (CMAP) amplitude, inhibited axonal regrowth, slowed M1 macrophage infiltration and shift to M2 phenotype, and delayed myelin debris clearance. Notably, all aforementioned results caused by KIF5b overexpression were alleviated by ropivacaine. Furthermore, we demonstrated that inhibition of Nav1.8 transport by A-803467 produced mitigating effects on the impairment of regenerative capacity induced by KIF5b overexpression similar to ropivacaine. These results suggest that ropivacaine promotes axonal regeneration at least partially by inhibiting KIF5b-mediated Nav1.8 forward transport.
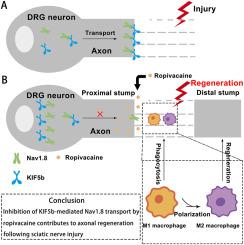
罗哌卡因抑制 KIF5b 介导的 Nav1.8 转运有助于大鼠坐骨神经损伤后的轴突再生。
周围神经损伤(PNI)通常由创伤性事故或医疗事件引起,是目前导致肢体残疾的最常见疾病之一。周围神经损伤后,耐河豚毒素的电压门控钠通道 Nav1.8 在损伤部位上调。我们早前的研究表明,罗哌卡因可通过调节 Nav1.8 介导的巨噬细胞信号来促进轴突再生。然而,罗哌卡因调控 Nav1.8 表达的机制仍不完全清楚。据报道,驱动蛋白家族 5b (KIF5b)介导了 Nav1.8 从背根神经节(DRGs)到病变部位的轴突运输。在此,我们研究了罗哌卡因是否能通过抑制 KIF5b 介导的 Nav1.8 转运促进轴突再生。KIF5b和Nav1.8在DRG中的水平降低与它们在病变部位的水平升高相吻合。Nav1.8 mRNA 在病变部位显著增加,但在 DRG 中却没有增加。令人惊讶的是,罗哌卡因逆转了 Nav1.8 和 KIF5b 蛋白表达的改变,但不影响 Nav1.8 mRNA 水平。由于 KIF5b 在 DRGs 中过表达,Nav1.8 蛋白水平在 DRGs 中显著降低,而在病变部位则显著升高。我们还发现,KIF5b过表达会明显损害行为功能,降低复合肌动作电位(CMAP)振幅的恢复指数,抑制轴突再生,减缓M1巨噬细胞浸润和向M2表型的转变,延迟髓鞘碎片的清除。值得注意的是,罗哌卡因能缓解 KIF5b 过表达引起的所有上述结果。此外,我们还证明,A-803467抑制Nav1.8转运对KIF5b过表达引起的再生能力损害的缓解作用与罗哌卡因相似。这些结果表明,罗哌卡因至少部分是通过抑制 KIF5b 介导的 Nav1.8 转运来促进轴突再生的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


