Extragonadal function of follicle-stimulating hormone: Evidence for a role in endothelial physiology and dysfunction
IF 3.8
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Aims
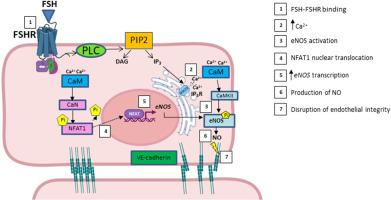
Follicle-stimulating hormone (FSH) plays a fundamental role in reproduction stimulating ovarian folliculogenesis, Sertoli cells function and spermatogenesis. However, the recent identification of FSH receptor (FSHR) also in extra-gonadal tissues has suggested that FSH activity may not be limited only to fertility regulation, with conflicting results on the possible role of FSH in endothelial cells. The aim of this study was to investigate FSH role on endothelial function in Human Umbilical Vein Endothelial Cells (HUVECs).
Results
Endothelial Nitric oxide synthase (eNOS) expression, eNOS phosphorylation and Nitric Oxide (NO) production resulted increased after the stimulation of HUVEC with recombinant human FSH (rhFSH) at 3.6x103 ng/ml, with increasing Calcium release from intracellular stores. Furthermore, IP3 production increased after rhFSH stimulation despite PTX treatment and NFAT1 was observed prevalently in nucleus.
We observed a statistical difference between untreated cells and cells stimulated with 0.36x103 ng/ml and between cells stimulated with 0.36x103 ng/ml and cells stimulated with 1.8x103 ng/ml at 4 and 8 h by Wound healing assay, respectively. Furthermore, a higher cellular permeability was observed in stimulated cells, with atypical VE-cadherin distribution, as well as filamentous actin.
Conclusions
Our findings suggest that FSH at high concentrations elicits a signalling that could compromise the endothelial membrane. Indeed, VE-cadherin anomalies may severely affect the endothelial barrier, resulting in an increased membrane permeability. Although NO is an important vasodilatation factor, probably an excessive production could impact on endothelial functionality, partially explaining the increased risk of cardiovascular diseases in menopausal women and men with hypogonadism.
卵泡刺激素的激素外功能:在内皮生理和功能障碍中发挥作用的证据。
目的:卵泡刺激素(FSH)在生殖过程中发挥着刺激卵巢卵泡生成、Sertoli细胞功能和精子生成的基本作用。然而,最近在性腺外组织中也发现了前列腺素受体(FSHR),这表明前列腺素的活性可能不仅限于生育调节,而关于前列腺素在血管内皮细胞中可能发挥的作用,结果却相互矛盾。本研究旨在探讨 FSH 对人脐静脉内皮细胞(HUVECs)内皮功能的作用:结果:用 3.6x103 纳克/毫升的重组人 FSH(rhFSH)刺激 HUVEC 后,内皮一氧化氮合酶(eNOS)表达、eNOS 磷酸化和一氧化氮(NO)产生均增加,细胞内储存的钙释放增加。此外,尽管有 PTX 处理,但在 rhFSH 刺激后 IP3 的产生仍有所增加,而且在细胞核中普遍观察到 NFAT1。通过伤口愈合试验,我们观察到未处理的细胞与受到 0.36x103 ng/ml 刺激的细胞之间,以及受到 0.36x103 ng/ml 刺激的细胞与受到 1.8x103 ng/ml 刺激的细胞之间,在 4 小时和 8 小时内分别存在统计学差异。此外,在受刺激的细胞中观察到更高的细胞通透性,VE-cadherin和丝状肌动蛋白分布不典型:我们的研究结果表明,高浓度的 FSH 可诱发损害内皮膜的信号。事实上,VE-cadherin 异常可能会严重影响内皮屏障,导致膜通透性增加。虽然一氧化氮是一种重要的血管扩张因子,但其过量产生可能会影响内皮功能,从而部分解释了更年期妇女和性腺功能减退症男性罹患心血管疾病风险增加的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


