Exploration of potential drug targets for Glaucoma by plasma proteome screening
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Background
Glaucoma is the leading cause of irreversible blindness. However, the current available treatment methods are still unsatisfactory. Therefore, the exploration of new drug targets for the treatment of glaucoma is of paramount importance.
Methods
We conducted two-sample Mendelian randomization (MR) using plasma protein quantitative trait loci (pQTL) data from two datasets (n = 734, n = 4907) and their instrumental variables to investigate the causal relationship between plasma proteins and glaucoma. The analysis was validated by replacing the exposure and outcome cohorts. Additionally, we utilized protein-protein interaction networks to assess the associations between these potential drug targets and existing drug targets.
Results
Through two-sample Mendelian randomization analysis, we identified causal relationships between Glaucoma and the following proteins: AZU1, OBP2B, ENPP5, INPP5B, KREMEN1, LYPLAL1, and PTPRJ. External validation confirmed the protective effect of LYPLAL1 on Glaucoma, while ENPP5, KREMEN1, and PTPRJ increased the risk of Glaucoma. Reverse MR and Steiger filtering did not indicate any reverse causal associations of the aforementioned proteins with Glaucoma.
Conclusion
Our study demonstrates a causal impact of ENPP5, KREMEN1, PTPRJ, and LYPLAL1 on the risk of Glaucoma. These findings suggest that these four proteins may serve as promising drug targets for Glaucoma treatment.
Significance
Currently, the pharmacological treatment of glaucoma primarily focuses on lowering intraocular pressure, which has its limitations. Targeted therapy is a personalized treatment approach that aims to inhibit or block the development and progression of diseases such as cancer and inflammation by selectively acting on specific biomolecules or signaling pathways. Our research employs a two-sample Mendelian randomization (MR) method, integrating a large amount of GWAS and pQTL data to perform MR analysis. This has enabled us to explore several plasma proteins as potential drug targets for glaucoma, providing direction and a research foundation for future investigations into glaucoma drug targets.
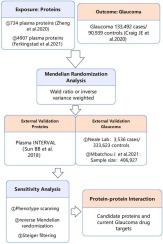
通过血浆蛋白质组筛选探索治疗青光眼的潜在药物靶点。
背景:青光眼是导致不可逆失明的主要原因。然而,目前可用的治疗方法仍不尽人意。因此,探索治疗青光眼的新药靶点至关重要:我们使用两个数据集(n = 734、n = 4907)中的血浆蛋白定量性状位点(pQTL)数据及其工具变量进行了双样本孟德尔随机化(MR),以研究血浆蛋白与青光眼之间的因果关系。通过替换暴露组群和结果组群,对分析进行了验证。此外,我们还利用蛋白质-蛋白质相互作用网络来评估这些潜在药物靶点与现有药物靶点之间的关联:通过双样本孟德尔随机分析,我们确定了青光眼与以下蛋白质之间的因果关系:AZU1、OBP2B、ENPP5、INPP5B、KREMEN1、LYPLAL1 和 PTPRJ。外部验证证实了 LYPLAL1 对青光眼的保护作用,而 ENPP5、KREMEN1 和 PTPRJ 会增加青光眼的风险。反向MR和Steiger过滤没有发现上述蛋白质与青光眼有任何反向因果关系:我们的研究表明,ENPP5、KREMEN1、PTPRJ 和 LYPLAL1 对青光眼风险有因果关系。这些发现表明,这四种蛋白质可能是治疗青光眼的有前途的药物靶点:目前,青光眼的药物治疗主要集中在降低眼压上,这有其局限性。靶向治疗是一种个性化的治疗方法,旨在通过选择性地作用于特定的生物大分子或信号通路,抑制或阻断癌症和炎症等疾病的发生和发展。我们的研究采用了双样本孟德尔随机化(MR)方法,整合了大量 GWAS 和 pQTL 数据来进行 MR 分析。这使我们能够探索几种血浆蛋白作为治疗青光眼的潜在药物靶点,为今后研究青光眼药物靶点提供了方向和研究基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


