Oral viscous budesonide solution for enhanced localized treatment of eosinophilic esophagitis through improved mucoadhesion and permeation
IF 3.7
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
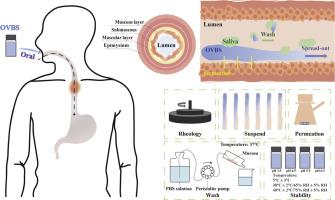
Eosinophilic esophagitis (EoE) is a chronic inflammatory disease of the esophagus that is immune/antigen-mediated and often requires targeted treatment. In clinical practice, an oral viscous budesonide suspension prepared by adding sucralose to a budesonide suspension for inhalation (Pulmicort®) is used to treat adult EoE and enhance retention in the esophageal mucosa. Inspired by this off-label drug use, oral viscous budesonide solutions (OVBSs) were developed in this study, and their capacities for adhesion, permeation, and stability were explored. Given the insolubility of budesonide as a BCS II drug, we first evaluated its equilibrium solubility and found that Transcutol® HP was an excellent choice for creating an OVBS at a concentration of 0.2 mg/g. The rheological properties of the OVBSs were evaluated with a rheometer, and shear-thinning, which aids in swallowing, was observed. The addition of hydroxyethyl cellulose (HEC) increased the adhesion strength of the preparation, which was associated with the hydration and thickening mechanism. This result was confirmed in a dynamic gelation study and in vitro elution experiment conducted with porcine esophagus tissue. Furthermore, the permeabilities of the OVBSs in the porcine esophagus were evaluated with a Franz diffusion cell device. >80 % of the budesonide was released after 24 h, and the release profile was similar to that of the solution. To explore the storage conditions of OVBSs, critical factors such as pH, content, and impurities were determined. It was found that OVBSs exhibited different behaviors at different pH values and temperatures. Notably, the OVBSs containing 1.7 % HEC could be stored for >6 months at a temperature of 5 °C ± 3 °C and a pH of 4.5 without significant degradation. Overall, this study demonstrated that OVBSs have the potential to adhere to the esophageal mucosa, permeate the tissue, and remain stable during storage. Moreover, OVBSs exhibit a distinct advantage over traditional converted inhalation-to-oral budesonide therapies by enabling flexible dose adjustment in clinical applications, thereby potentially minimizing systemic side effects commonly associated with oral glucocorticoid administration.
粘稠布地奈德口服溶液通过改善粘附性和渗透性加强对嗜酸性粒细胞食管炎的局部治疗
嗜酸性粒细胞食管炎(EoE)是一种食管慢性炎症性疾病,由免疫/抗原介导,通常需要针对性治疗。在临床实践中,一种通过在吸入用布地奈德混悬液(Pulmicort®)中添加蔗糖而制备的粘稠布地奈德口服混悬液被用于治疗成人食管炎,并增强其在食管粘膜中的滞留。受这种标签外用药的启发,本研究开发了口服粘稠布地奈德溶液(OVBSs),并探索了其粘附、渗透和稳定性能力。鉴于布地奈德作为一种 BCS II 药物具有不溶性,我们首先对其平衡溶解度进行了评估,发现 Transcutol® HP 是配制浓度为 0.2 mg/g 的 OVBS 的最佳选择。我们使用流变仪对 OVBS 的流变特性进行了评估,观察到了有助于吞咽的剪切稀化现象。羟乙基纤维素(HEC)的加入增加了制剂的粘附强度,这与水合和增稠机制有关。用猪食道组织进行的动态凝胶研究和体外洗脱实验证实了这一结果。此外,还利用弗朗兹扩散池装置评估了 OVBS 在猪食道中的渗透性。24 小时后,超过 80% 的布地奈德被释放出来,释放曲线与溶液相似。为了探索 OVBS 的储存条件,研究人员测定了 pH 值、含量和杂质等关键因素。结果发现,OVBS 在不同的 pH 值和温度下表现出不同的行为。值得注意的是,含有 1.7% HEC 的 OVBS 在温度为 5°C ± 3°C 和 pH 值为 4.5 的条件下可储存 6 个月以上,且无明显降解。总之,这项研究表明,OVBSs 具有粘附食管粘膜、渗透组织并在储存期间保持稳定的潜力。此外,与传统的吸入-口服布地奈德转换疗法相比,OVBSs 具有明显的优势,在临床应用中可以灵活调整剂量,从而有可能最大限度地减少口服糖皮质激素常见的全身副作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.30
自引率
13.20%
发文量
367
审稿时长
33 days
期刊介绍:
The Journal of Pharmaceutical Sciences will publish original research papers, original research notes, invited topical reviews (including Minireviews), and editorial commentary and news. The area of focus shall be concepts in basic pharmaceutical science and such topics as chemical processing of pharmaceuticals, including crystallization, lyophilization, chemical stability of drugs, pharmacokinetics, biopharmaceutics, pharmacodynamics, pro-drug developments, metabolic disposition of bioactive agents, dosage form design, protein-peptide chemistry and biotechnology specifically as these relate to pharmaceutical technology, and targeted drug delivery.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


