Suppression of matrigel-induced choroidal neovascularization by AAV delivery of a novel anti-Scg3 antibody
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Efforts to develop gene therapy for long-term treatment of neovascular disease are hampered by ongoing concerns that biologics against vascular endothelial growth factor (VEGF) inhibit both physiological and pathological angiogenesis and are therefore at elevated risk of adverse side effects. A potential solution is to develop disease-targeted gene therapy. Secretogranin III (Scg3), a unique disease-restricted angiogenic factor described by our group, contributes significantly to ocular neovascular disease. We have shown that Scg3 blockade with a monoclonal antibody Fab fragment (Fab) stringently inhibits pathological angiogenesis without affecting healthy vessels. Here we tested the therapeutic efficacy of adeno-associated virus (AAV)-anti-Scg3Fab to block choroidal neovascularization (CNV) induced by subretinal injection of Matrigel in a mouse model. Intravitreal AAV-anti-Scg3Fab significantly reduced CNV and suppressed CNV-associated leukocyte infiltration and macrophage activation. The efficacy and anti-inflammatory effects were equivalent to those achieved by positive control AAV-aflibercept against VEGF. Efficacies of AAV-anti-Scg3Fab and AAV-aflibercept were sustained over 4 months post AAV delivery. The findings support development of AAV-anti-Scg3 as an alternative to AAV-anti-VEGF with equivalent efficacy and potentially safer mechanism of action.
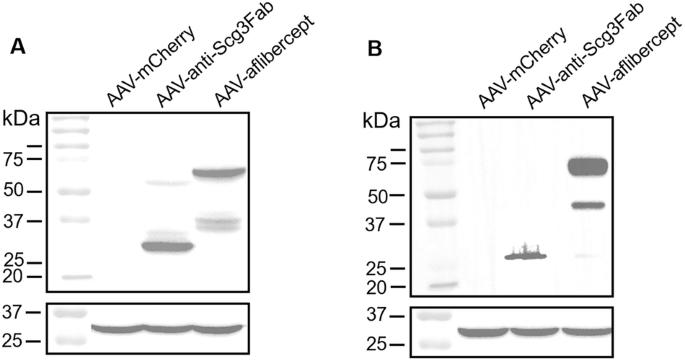
通过 AAV 释放新型抗 Scg3 抗体抑制 matrigel 诱导的脉络膜新生血管。
人们一直担心,针对血管内皮生长因子(VEGF)的生物制剂会抑制生理性和病理性血管生成,因此产生不良副作用的风险较高,这阻碍了开发用于长期治疗新生血管疾病的基因疗法的努力。一种潜在的解决方案是开发疾病靶向基因疗法。Secretogranin III(Scg3)是我们研究小组发现的一种独特的疾病限制性血管生成因子,对眼部新生血管疾病有重要作用。我们已经证明,用单克隆抗体 Fab 片段(Fab)阻断 Scg3 能严格抑制病理性血管生成,而不影响健康血管。在此,我们测试了腺相关病毒(AAV)-抗-Scg3Fab 在小鼠模型中阻断由视网膜下注射 Matrigel 诱导的脉络膜新生血管(CNV)的疗效。玻璃体内AAV-抗-Scg3Fab能显著减少CNV,抑制CNV相关的白细胞浸润和巨噬细胞活化。其疗效和抗炎作用与抗血管内皮生长因子的阳性对照 AAV-aflibercept 所达到的效果相当。AAV-抗Scg3Fab和AAV-aflibercept的疗效可在AAV递送后4个月内持续。研究结果支持开发AAV-抗Scg3,作为AAV-抗VEGF的替代品,其疗效相当,作用机制可能更安全。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


