MLKL-mediated endothelial necroptosis drives vascular damage and mortality in systemic inflammatory response syndrome
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
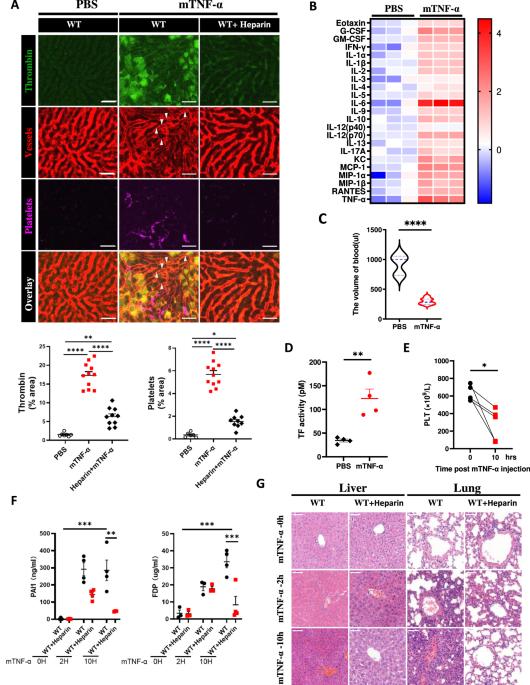
The hypersecretion of cytokines triggers life-threatening systemic inflammatory response syndrome (SIRS), leading to multiple organ dysfunction syndrome (MODS) and mortality. Although both coagulopathy and necroptosis have been identified as important factors in the pathogenesis of SIRS, the specific cell types that undergo necroptosis and the interrelationships between coagulopathy and necroptosis remain unclear. In this study, we utilized visualization analysis via intravital microscopy to demonstrate that both anticoagulant heparin and nonanticoagulant heparin (NAH) pretreatment protect mice against TNF-α-induced mortality in SIRS. Moreover, the deletion of Mlkl or Ripk3 resulted in decreased coagulation and reduced mortality in TNF-α-induced SIRS. These findings suggest that necroptosis plays a key role upstream of coagulation in SIRS-related mortality. Furthermore, using a genetic lineage tracing mouse model (Tie2-Cre;Rosa26-tdT), we tracked endothelial cells (ECs) and verified that EC necroptosis is responsible for the vascular damage observed in TNF-α-treated mice. Importantly, Mlkl deletion in vascular ECs in mice had a similar protective effect against lethal SIRS by blocking EC necroptosis to protect the integrity of the endothelium. Collectively, our findings demonstrated that RIPK3–MLKL-dependent necroptosis disrupted vascular integrity, resulting in coagulopathy and multiorgan failure, eventually leading to mortality in SIRS patients. These results highlight the importance of targeting vascular EC necroptosis for the development of effective treatments for SIRS patients.
MLKL 介导的内皮坏死促使全身炎症反应综合征的血管损伤和死亡。
细胞因子分泌过多会引发危及生命的全身炎症反应综合征(SIRS),导致多器官功能障碍综合征(MODS)和死亡。虽然凝血病变和坏死都被认为是 SIRS 发病机制中的重要因素,但发生坏死的特定细胞类型以及凝血病变和坏死之间的相互关系仍不清楚。在本研究中,我们利用体视显微镜进行了可视化分析,证明抗凝肝素和非抗凝肝素(NAH)预处理都能保护小鼠免受 TNF-α 诱导的 SIRS 死亡率的影响。此外,在 TNF-α 诱导的 SIRS 中,Mlkl 或 Ripk3 的缺失导致凝血功能下降,死亡率降低。这些研究结果表明,在与 SIRS 相关的死亡率中,坏死蛋白在凝血的上游起着关键作用。此外,我们利用遗传系谱追踪小鼠模型(Tie2-Cre;Rosa26-tdT)追踪了内皮细胞(ECs),并验证了EC坏死是 TNF-α 处理小鼠血管损伤的原因。重要的是,小鼠血管内皮细胞中的 Mlkl 基因缺失通过阻断内皮细胞坏死以保护内皮的完整性,对致命的 SIRS 具有类似的保护作用。总之,我们的研究结果表明,RIPK3-MLKL 依赖性坏死破坏了血管完整性,导致凝血功能障碍和多器官功能衰竭,最终导致 SIRS 患者死亡。这些结果凸显了针对血管内皮细胞坏死开发有效治疗 SIRS 患者方法的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.
文献相关原料
公司名称
产品信息
阿拉丁
formamide

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


