High activity of the new myeloablative regimen of gemcitabine/clofarabine/busulfan for allogeneic transplant for aggressive lymphomas
IF 4.5
2区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Refractory aggressive lymphomas can be treated with allo-SCT, pursuing a graft-vs-lymphoma effect. While reduced intensity conditioning is safe, tumors often progress rapidly, indicating the need for more active conditioning regimens. The preclinical synergy we saw between gemcitabine (Gem), clofarabine (Clo) and busulfan (Bu) against lymphoma cell lines led us to study Gem/Clo/Bu clinically. Eligibility: age 12–65, refractory aggressive B-NHL, T-NHL or Hodgkin, with a matched donor. Infusional Gem was dose-escalated on days (d) −6 and −4 (475–975 mg/m2/day), followed by Clo (40 mg/m2/day) and Bu (target AUC, 4000 μMol min/day) (d −6 to −3). CD20+ tumors received rituximab. GVHD prophylaxis included ATG (MUD), tacrolimus and MMF. We compared their outcomes to matched-pair concurrent controls receiving Flu/Mel + matched allo-SCT. We enrolled 64 patients, median age 46 (17–63), 31 B-NHL/22 T-NHL/11 Hodgkin, 36 MSD/28 MUD (all PBPC), median 4 (2–10) prior therapies; 18 prior auto-SCT, 42 active diseases at allo-SCT (12 PD). Toxicities (mucositis and transaminitis) were manageable. Gem/Clo/Bu was myeloablative yielding early full donor chimerism. Grades II–IV/III–IV acute GVHD rates of 37% and 18%; chronic GVHD of 33% (13% severe); NRM at D100/1 year was 7% and 18%. ORR/CR rates: 78%/71% (B-NHL), 93%/93% (T-NHL), 67%/67% (Hodgkin). At a median follow-up of 60 (12–110) months, EFS/OS rates: 36%/47%. Gem/Clo/Bu patients had better median EFS (12 vs. 3 months, P = 0.001) and OS (25 vs. 7 months, P = 0.003) than 113 Flu/Mel matched-pair controls. The new myeloablative regimen Gem/Clo/Bu has limited toxicity and high activity in allo-SCT for aggressive lymphomas, yielding better outcomes than concurrent matched-pair controls receiving Flu/Mel.
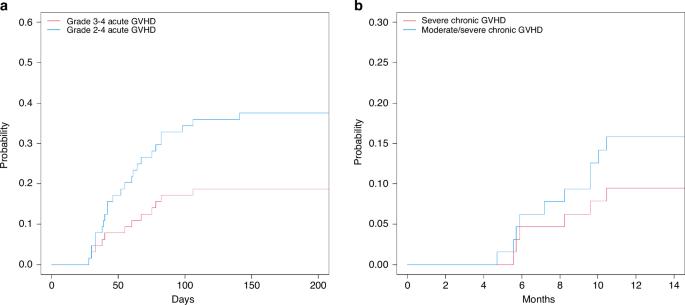
吉西他滨/氯法拉滨/布舒凡的新髓鞘消融方案在侵袭性淋巴瘤异基因移植中的高活性。
难治性侵袭性淋巴瘤可通过异体造血干细胞移植治疗,追求移植物-淋巴瘤效应。虽然降低强度的调理是安全的,但肿瘤往往进展迅速,这表明需要更积极的调理方案。我们在临床前发现吉西他滨(Gem)、氯法拉滨(Clo)和丁硫(Bu)对淋巴瘤细胞株有协同作用,这促使我们对 Gem/Clo/Bu 进行临床研究。研究对象:12-65 岁,难治性侵袭性 B-NHL、T-NHL 或霍奇金淋巴瘤,有匹配的供体。输注 Gem 的剂量在第 -6 天和第 -4 天递增(475-975 毫克/平方米/天),然后是 Clo(40 毫克/平方米/天)和 Bu(目标 AUC,4000 μMol min/天)(第 -6 天至第 -3 天)。CD20+肿瘤接受利妥昔单抗治疗。GVHD 预防药物包括 ATG (MUD)、他克莫司和 MMF。我们将他们的治疗结果与同时接受 Flu/Mel + 匹配异体干细胞移植的配对对照组进行了比较。我们共招募了 64 名患者,中位年龄为 46 岁(17-63 岁),31 名 B-NHL/22 名 T-NHL/11 名霍奇金,36 名 MSD/28 名 MUD(均为 PBPC),中位数为 4(2-10)次既往治疗;18 名既往接受过自身 SCT,42 名在接受 allo-SCT 时疾病处于活动期(12 名 PD)。毒性(粘膜炎和转氨酶)可控。Gem/Clo/Bu是一种髓鞘消融疗法,能在早期产生完全的供体嵌合体。II-IV/III-IV级急性GVHD发生率分别为37%和18%;慢性GVHD发生率为33%(13%为重度);D100/1年的NRM分别为7%和18%。ORR/CR率:ORR/CR率:78%/71%(B-NHL)、93%/93%(T-NHL)、67%/67%(霍奇金)。中位随访时间为60(12-110)个月,EFS/OS率为36%/47%:36%/47%.Gem/Clo/Bu 患者的中位 EFS(12 个月对 3 个月,P = 0.001)和 OS(25 个月对 7 个月,P = 0.003)均优于 113 Flu/Mel 配对对照组。与同时接受Flu/Mel的配对对照组相比,新的骨髓溶解疗法Gem/Clo/Bu在侵袭性淋巴瘤的异体造血干细胞移植中毒性有限,活性高,疗效更好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bone Marrow Transplantation
医学-免疫学
CiteScore
8.40
自引率
8.30%
发文量
337
审稿时长
6 months
期刊介绍:
Bone Marrow Transplantation publishes high quality, peer reviewed original research that addresses all aspects of basic biology and clinical use of haemopoietic stem cell transplantation.
The broad scope of the journal thus encompasses topics such as stem cell biology, e.g., kinetics and cytokine control, transplantation immunology e.g., HLA and matching techniques, translational research, and clinical results of specific transplant protocols. Bone Marrow Transplantation publishes 24 issues a year.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


