Characterization of PARP1 binding to c-KIT1 G-quadruplex DNA: Insights into domain-specific interactions
IF 3.3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Poly(ADP-ribose) polymerase 1 (PARP1) is a nuclear enzyme involved in catalyzing Poly-(ADP-ribosyl)ation. PARP1 binds to different forms of DNA and DNA breaks and thus plays important roles in several cellular processes, including DNA damage repair, cell cycle regulation, chromatin remodeling, and maintaining genomic stability. In this study, we conducted biochemical and biophysical characterization of PARP1 binding to G-quadruplex DNA (G4-DNA). Our investigation identified ZnF1, ZnF3, and WGR as the critical domains to mediate PARP1 binding to G4-c-KIT1. Also, our results show that these domains together show cooperativity for G4-c-KIT1 recognition. Further, we establish that the presence of an oxidized (5-carboxylcytosine) base in the loop region of G4-c-KIT1 (G4-5caC-cKIT1) does not affect its recognition by PARP1. Both G4-c-KIT1 and G4-5caC-cKIT1 are potent stimulators of PARP1's catalytic activity. Our study advances the understanding of PARP1's versatile DNA binding capabilities for G4-c-KIT1 DNA irrespective of the oxidation/ modification in the DNA base. These insights into PARP1's domain-specific contributions to G4-c-KIT1 DNA recognition and catalysis expand our knowledge of its multifaceted roles in DNA repair and genome maintenance.
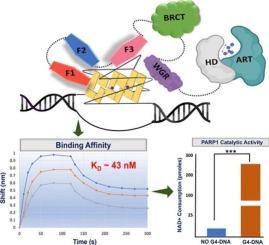
PARP1 与 c-KIT1 G-quadruplex DNA 结合的特征:洞察特定结构域的相互作用。
聚(ADP-核糖)聚合酶 1(PARP1)是一种参与催化聚(ADP-核糖)合成的核酶。PARP1 与不同形式的 DNA 和 DNA 断裂结合,因此在 DNA 损伤修复、细胞周期调控、染色质重塑和维持基因组稳定性等多个细胞过程中发挥着重要作用。在这项研究中,我们对 PARP1 与 G 型四倍体 DNA(G4-DNA)的结合进行了生化和生物物理鉴定。我们的研究发现 ZnF1、ZnF3 和 WGR 是介导 PARP1 与 G4-c-KIT1 结合的关键结构域。同时,我们的研究结果表明,这些结构域在 G4-c-KIT1 识别过程中具有协同作用。此外,我们还证实,G4-c-KIT1(G4-5caC-KIT1)的环状区域中存在氧化(5-羧基胞嘧啶)碱基不会影响 PARP1 对其的识别。G4-c-KIT1 和 G4-5caC-cKIT1 都能有效刺激 PARP1 的催化活性。我们的研究加深了人们对 PARP1 与 G4-c-KIT1 DNA 的多功能 DNA 结合能力的理解,无论 DNA 碱基的氧化/修饰情况如何。这些关于 PARP1 对 G4-c-KIT1 DNA 识别和催化的特异领域贡献的见解,拓展了我们对其在 DNA 修复和基因组维护中的多方面作用的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


