Identification of dystrophin Dp71dΔ71-associated proteins in PC12 cells by quantitative proteomics
IF 2.3
4区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Proteins and proteomics
Pub Date : 2024-09-28
DOI:10.1016/j.bbapap.2024.141049
引用次数: 0
Abstract
Dystrophin Dp71 is essential for the development of the nervous system. Its alteration is associated with intellectual disability. Different Dp71 isoforms are generated by alternative splicing; however, their functions have not been fully described. Here, we identified Dp71dΔ71-associated proteins to understand the complex functions. PC12 cells, stably transfected with pTRE2pur-Myc/Dp71dΔ71 or pTRE2pur-Myc empty vector (EV), were analyzed by immunoprecipitation followed with quantitative proteomics with data-independent acquisition and ion mobility separation. We used the Top3 method to quantify absolutely every protein detected. A total of 106 proteins were quantified with Progenesis QI software and the database UP000002494. Seven new proteins associated with Dp71dΔ71 were selected with at least 2-fold quantity between immunoprecipitated proteins of PC12-Myc/Dp71dΔ71 versus PC12-EV cells. These results revealed new proteins that interact with Dp71dΔ71, including β-Tubulin, S-adenosylmethionine synthase isoform type-2, adapter molecule crk, helicase with zinc finger 2, WD repeat domain 93, cyclin-L2 and myosin-10, which are related to cell migration and/or cell growth. The results lay the foundation for future research on the relationship between these proteins and Dp71 isoforms.
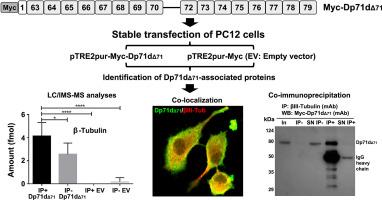
通过定量蛋白质组学鉴定 PC12 细胞中的肌营养不良蛋白 Dp71dΔ71 相关蛋白。
肌营养不良蛋白 Dp71 对神经系统的发育至关重要。它的改变与智力残疾有关。不同的 Dp71 异构体通过替代剪接产生,但它们的功能尚未得到充分描述。在此,我们鉴定了 Dp71dΔ71 相关蛋白,以了解其复杂的功能。PC12 细胞经 pTRE2pur-Myc/Dp71dΔ71 或 pTRE2pur-Myc 空载体 (EV) 稳定转染后,通过免疫共沉淀结合定量蛋白质组学和超高效液相色谱 ACQUITY M-Class 进行分析。质谱数据是在电喷雾离子化和离子迁移率分离的 Synapt G2-Si 质谱仪上获得的,该质谱仪采用独立数据采集和离子迁移率质谱,使用高清晰度多路复用 MS/MS 模式。我们使用 Hi3 方法对检测到的所有蛋白质进行量化。使用 Progenesis QI 软件和数据库 UP000002494 共对 121 个蛋白质进行了量化。在 PC12-Myc/Dp71dΔ71 与 PC12-EV 细胞的免疫沉淀蛋白中,筛选出了 7 个与 Dp71dΔ71 相关的新蛋白,其数量至少是 PC12-Myc/Dp71dΔ71 与 PC12-EV 细胞的 2 倍。这些结果揭示了与Dp71dΔ71相互作用的新蛋白,包括β-微管蛋白、S-腺苷蛋氨酸合成酶同工型-2、适配器分子crk、带锌指的螺旋酶2、WD重复结构域93、细胞周期蛋白-L2和肌球蛋白-10,它们与细胞迁移和/或细胞生长有关。这些结果为今后研究这些蛋白与 Dp71 同工酶之间的关系奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
0.00%
发文量
55
审稿时长
33 days
期刊介绍:
BBA Proteins and Proteomics covers protein structure conformation and dynamics; protein folding; protein-ligand interactions; enzyme mechanisms, models and kinetics; protein physical properties and spectroscopy; and proteomics and bioinformatics analyses of protein structure, protein function, or protein regulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


