Endoplasmic Reticulum Stress Delays Choroid Development in the HCAR1 Knockout Mouse
IF 4.7
2区 医学
Q1 PATHOLOGY
引用次数: 0
Abstract
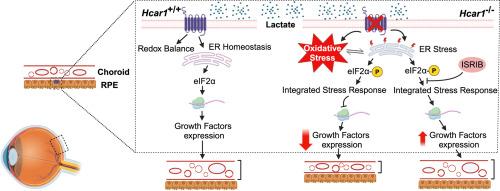
The subretina, composed of the choroid and the retinal pigment epithelium (RPE), plays a critical role in proper vision. In addition to phagocytosis of photoreceptor debris, the RPE shuttles oxygen and nutrients to the neuroretina. For their own energy production, RPE cells mainly rely on lactate, a major by-product of glycolysis. Lactate, in turn, conveys most of its biological effects via the hydroxycarboxylic acid receptor 1 (HCAR1). Herein, the lactate-specific receptor, HCAR1, was found to be exclusively expressed in the RPE cells within the subretina, and Hcar1−/− mice exhibited a substantially thinner choroidal vasculature during development. Notably, the angiogenic properties of lactate on the choroid were impacted by the absence of Hcar1. HCAR1-deficient mice exhibited elevated endoplasmic reticulum stress along with eukaryotic translation initiation factor 2α phosphorylation, a significant decrease in the global protein translation rate, and a lower proliferation rate of choroidal vasculature. Strikingly, inhibition of the integrated stress response using an inhibitor that reverses the effect of eukaryotic translation initiation factor 2α phosphorylation restored protein translation and rescued choroidal thinning. These results provide evidence that lactate signalling via HCAR1 is important for choroidal development/angiogenesis and highlight the importance of this receptor in establishing mature vision.
内质网应激导致 HCAR1 基因敲除小鼠脉络膜发育延迟
视网膜下由脉络膜和视网膜色素上皮(RPE)组成,对正常视觉起着至关重要的作用。除了吞噬光感受器碎片外,RPE 还将氧气和营养物质输送到神经视网膜。RPE 细胞自身产生能量主要依靠乳酸,这是糖酵解的一种主要副产品。而乳酸被认为是通过羟基羧酸受体 1(HCAR1)传递其大部分生物效应的。研究发现,乳酸特异性受体 HCAR1 只在视网膜下的 RPE 细胞中表达,而 Hcar1-/- 小鼠在发育过程中脉络膜血管明显变细。值得注意的是,乳酸对脉络膜的血管生成特性会因 Hcar1 的缺失而受到影响。缺乏 HCAR1 的小鼠表现出内质网应激升高,真核起始因子 2α 磷酸化,整体蛋白质翻译率显著下降,脉络膜血管增殖率降低。令人震惊的是,使用能逆转真核细胞启动因子 2α 磷酸化效应的抑制剂抑制综合应激反应,可恢复蛋白质翻译并挽救脉络膜变薄。这些结果提供了证据,证明通过 HCAR1 发出的乳酸信号对脉络膜的发育/血管生成很重要,并强调了该受体在建立成熟视觉中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


