Synthesis and biological evaluation of ferrostatin-based diamide derivatives as new ferroptosis inhibitors
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Ferroptosis, a distinct type of cell death caused by iron and lipid peroxidation, has been associated with several diseases, including cardiovascular disorders. Ferrostatin-1 (Fer-1) is a known ferroptosis inhibitor, but its clinical application is limited by low efficacy and stability. In the present study, a series of Fer-1-based diamide derivatives was synthesized and evaluated to enhance ferroptosis inhibition and in vitro metabolic stability. The synthesized compounds were tested for their protective effects against Erastin-induced injury in human vascular endothelial cells (HUVECs). Among the derivatives, compound 36 exhibited the most potent anti-ferroptosis activity with an EC50 value of 0.58 ± 0.02 µM. Remarkably, compound 36 also demonstrated superior stability in both microsomal (human and mouse) and mouse plasma assays. These findings indicated ferroptosis inhibitor 36 as a promising hit for further developing potential therapeutic drug candidates in cardiovascular diseases.
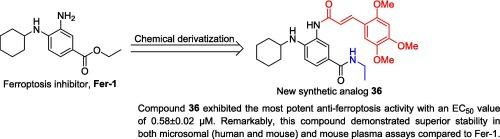
铁锈素类二酰胺衍生物的合成和生物学评价,作为新的铁锈素抑制剂。
铁氧化是一种由铁和脂质过氧化引起的独特细胞死亡类型,与包括心血管疾病在内的多种疾病有关。铁前列素-1(Fer-1)是一种已知的铁突变抑制剂,但其临床应用因药效低和稳定性差而受到限制。本研究合成了一系列以 Fer-1 为基础的二酰胺衍生物,并对其进行了评估,以增强其铁蛋白沉积抑制作用和体外代谢稳定性。测试了合成的化合物对 Erastin 诱导的人血管内皮细胞(HUVECs)损伤的保护作用。在这些衍生物中,化合物 36 的 EC50 值为 0.58 ± 0.02 µM,具有最强的抗铁丝蛋白沉积活性。值得注意的是,化合物 36 在微粒体(人和小鼠)和小鼠血浆试验中也表现出卓越的稳定性。这些研究结果表明,铁蛋白沉积抑制剂 36 是一种很有希望的新药,可用于进一步开发治疗心血管疾病的潜在候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


