Design, synthesis and antitumor activity of novel 4-oxobutanamide derivatives
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
To find highly effective and low-toxicity antitumor drugs to overcome the challenge of cancer, we designed and synthesized a series of novel 4-oxobutanamide derivatives using the principle of molecular hybridization and tested the antiproliferative ability of the title compounds against human cervical carcinoma cells (HeLa), human breast carcinoma cells (MDA-MB-231) and human kidney carcinoma cells (A498). Among them, N1-(4-methoxybenzyl)-N4-(4-methoxyphenyl)-N1-(3,4,5-trimethoxyphenyl) succinimide DN4 (IC50 = 1.94 µM) showed the best proliferation activity on A498, superior to the positive control paclitaxel (IC50 = 8.81 µM) and colchicine (IC50 = 7.17 µM). Compound DN4 not only inhibited the proliferation, adhesion and invasion of A498, but also inhibited angiogenesis and tumor growth in a dose-dependent manner in the xenograft model of A498 cells. In addition, we also predicted the physicochemical properties and toxicity (ADMET) of these derivatives, and the results suggested that these derivatives may have the absorption, distribution, metabolism, excretion, and toxicity properties of drug candidates. Thus, compound DN4 may be a promising drug candidate for the treatment of cancer.
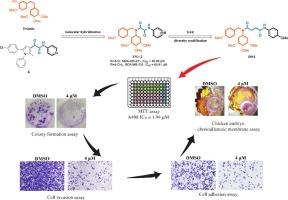
新型 4-氧代丁酰胺衍生物的设计、合成和抗肿瘤活性。
为了找到高效低毒的抗肿瘤药物来攻克癌症这一难题,我们利用分子杂交原理设计并合成了一系列新型 4-氧代丁酰胺衍生物,并测试了标题化合物对人宫颈癌细胞(HeLa)、人乳腺癌细胞(MDA-MB-231)和人肾癌细胞(A498)的抗增殖能力。其中,N1-(4-甲氧基苄基)-N4-(4-甲氧基苯基)-N1-(3,4,5-三甲氧基苯基)琥珀酰亚胺 DN4(IC50 = 1.94 µM)对 A498 的增殖活性最好,优于阳性对照紫杉醇(IC50 = 8.81 µM)和秋水仙碱(IC50 = 7.17 µM)。化合物 DN4 不仅能抑制 A498 的增殖、粘附和侵袭,还能在 A498 细胞异种移植模型中以剂量依赖的方式抑制血管生成和肿瘤生长。此外,我们还预测了这些衍生物的理化性质和毒性(ADMET),结果表明这些衍生物可能具有候选药物的吸收、分布、代谢、排泄和毒性特性。因此,化合物 DN4 可能是一种治疗癌症的有前途的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


