TMCO1 promotes ferroptosis and ECM deposition in glaucomatous trabecular meshwork via ERK1/2 signaling
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-09-27
DOI:10.1016/j.bbadis.2024.167530
引用次数: 0
Abstract
Glaucoma, a leading cause of global blindness, is marked by irreversible retinal ganglion cells (RGCs) loss, elevated intraocular pressure (IOP), and extracellular matrix (ECM) deposition in the trabecular meshwork (TM). Transmembrane and coiled-coil domain protein 1 (TMCO1), implicated in calcium regulation, has potential links to primary open-angle glaucoma (POAG). Ferroptosis, an iron-dependent cell death mechanism driven by lipid peroxidation, is also observed in glaucoma. This study investigates the role of TMCO1 in POAG, focusing on its involvement in TM ECM deposition via ferroptosis induction and ERK1/2 phosphorylation inhibition.
In both in vivo and in vitro models, we demonstrated that dexamethasone (DEX) stimulation upregulates TMCO1, leading to increased ECM deposition and ferroptosis in human trabecular meshwork cells (HTMCs). Furthermore, treatment with ferrostatin-1 (Fer-1), a ferroptosis inhibitor, significantly reduced ECM deposition and ferroptosis in HTMCs. These findings establish TMCO1 as a critical regulator of ferroptosis and ECM deposition through the ERK/MAPK pathway, positioning it as a promising therapeutic target for glaucoma.
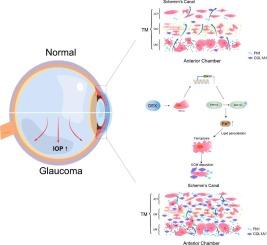
TMCO1 通过 ERK1/2 信号传导促进青光眼小梁网的铁蛋白沉积和 ECM 沉积。
青光眼是导致全球失明的主要原因之一,其特征是不可逆的视网膜神经节细胞(RGC)丧失、眼压(IOP)升高以及细胞外基质(ECM)在小梁网(TM)中沉积。跨膜和盘卷结构域蛋白 1 (TMCO1) 与钙调节有关,它与原发性开角型青光眼 (POAG) 有潜在的联系。在青光眼中也可观察到铁氧化,这是一种由脂质过氧化驱动的铁依赖性细胞死亡机制。本研究调查了 TMCO1 在 POAG 中的作用,重点研究其通过诱导铁蜕变和抑制 ERK1/2 磷酸化参与 TM ECM 沉积。在体内和体外模型中,我们证实地塞米松(DEX)刺激可上调 TMCO1,导致人小梁网状细胞(HTMCs)中的 ECM 沉积和铁蛋白沉积增加。此外,用铁蛋白抑制剂铁前列素-1(Fer-1)治疗可显著减少小梁网细胞中的 ECM 沉积和铁蛋白沉积。这些发现确定了 TMCO1 是通过 ERK/MAPK 通路调节铁蜕变和 ECM 沉积的关键因素,并将其定位为治疗青光眼的有望靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


