SENP1 prevents high fat diet-induced non-alcoholic fatty liver diseases by regulating mitochondrial dynamics
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-09-26
DOI:10.1016/j.bbadis.2024.167527
引用次数: 0
Abstract
Mitochondrial dynamics plays a crucial role in the occurrence and development of non-alcoholic fatty liver diseases (NAFLD). SENP1, a SUMO-specific protease, catalyzes protein de-SUMOylation and involves in various physiological and pathological processes. However, the exact role of SENP1 in NAFLD remains unclear. Therefore, we investigated the regulatory role of SENP1 in mitochondrial dynamics during the progression of NAFLD. In the study, the NAFLD in vivo model induced by high fat diet (HFD) and in vitro model induced by free fatty acids (FFA) were established to investigate the role and underlying mechanism of SENP1 through detecting mitochondrial morphology and dynamics. Our results showed that the down-regulation of SENP1 expression and the mitochondrial dynamics dysregulation occurred in the NAFLD, evidenced as mitochondrial fragmentation, up-regulation of p-Drp1 ser616 and down-regulation of MFN2, OPA1. However, over-expression of SENP1 significantly alleviated the NAFLD, rectified the mitochondrial dynamics disorder, reduced Cyt-c release and ROS levels induced by FFA or HFD; moreover, the over-expression of SENP1 also reduced the SUMOylation levels of Drp1 and prevented the Drp1 translocation to mitochondria. Our findings suggest that the possible mechanisms of SENP1 were through rectifying the mitochondrial dynamics disorder, reducing Cyt-c release and ROS-mediated oxidative stress. The findings would provide a novel target for the prevention and treatment of NALFD.
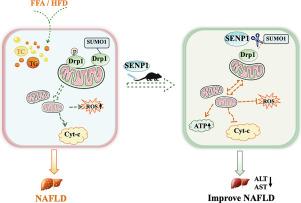
SENP1 通过调节线粒体动力学预防高脂饮食诱发的非酒精性脂肪肝。
线粒体动力学在非酒精性脂肪肝(NAFLD)的发生和发展中起着至关重要的作用。SENP1是一种SUMO特异性蛋白酶,可催化蛋白质去SUMO化,并参与各种生理和病理过程。然而,SENP1 在非酒精性脂肪肝中的确切作用仍不清楚。因此,我们研究了 SENP1 在非酒精性脂肪肝进展过程中对线粒体动力学的调控作用。研究建立了高脂饮食(HFD)诱导的非酒精性脂肪肝体内模型和游离脂肪酸(FFA)诱导的非酒精性脂肪肝体外模型,通过检测线粒体的形态和动态来研究 SENP1 的作用和内在机制。结果表明,在非酒精性脂肪肝中,SENP1表达下调,线粒体动力学失调,表现为线粒体破碎、p-Drp1 ser616上调、MFN2和OPA1下调。然而,过量表达 SENP1 能明显缓解非酒精性脂肪肝,纠正线粒体动力学紊乱,减少 FFA 或 HFD 诱导的 Cyt-c 释放和 ROS 水平;此外,过量表达 SENP1 还能降低 Drp1 的 SUMOylation 水平,阻止 Drp1 转位至线粒体。我们的研究结果表明,SENP1 的可能作用机制是通过纠正线粒体动力学紊乱、减少 Cyt-c 释放和 ROS 介导的氧化应激。这些发现将为预防和治疗 NALFD 提供一个新的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


