miR4352b a cross-species modulator of SOSTDC1, targets dual pathway to regulate bone health and fracture healing
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-09-24
DOI:10.1016/j.bbadis.2024.167514
引用次数: 0
Abstract
Mutations in SOST can lead to various monogenic bone diseases. Its paralog, SOSTDC1, shares 55 % protein sequence homology and belongs to the BMP antagonist class. Sostdc1−/− mice exhibit distinct effects on cortical and trabecular bone. Genetic polymorphisms in SOSTDC1 impacting peak bone mass makes SOSTDC1 gene, a candidate for influencing BMD variation in humans. SOSTDC1 is upregulated in bone loss conditions, altering BMP-responsive genes and signaling modulators, suggesting its dual BMP/Wnt antagonist role may enhance both pathways. Overexpression of SOSTDC1 confirmed its role as an osteogenic antagonist. Glycine max (Soy)-derived miR4352b, identified for cross-kingdom applications, precisely targets SOSTDC1, a key regulator of bone. SOSTDC1 competitively binds to BMP2 receptor, BMPR1A. Gma-miR4352b suppresses SOSTDC1 expression, enhancing osteogenesis and countering SOSTDC1's inhibition of osteogenic potential. Modeling estrogen deficiency to mimic elevated SOSTDC1 levels, we observed an inverse correlation with SOSTDC1 expression, while serum BMP2 and PINP levels increased following gma-miR4352b supplementation. In fracture healing, SOSTDC1's crucial role becomes evident in conditions of delayed fracture healing. As healing progresses, SOSTDC1 expression decreases. Gma-miR4352b, compared to scrambled miRNA, remarkably promotes callus formation, achieving 68 % healing by day 10, surpassing the scrambled group at 44 %. By the day 13, the treatment group exhibits advanced healing, challenging to find the callus, while the scrambled group maintains a healing rate similar to day10. The accelerated healing in the treatment group underscores the importance of SOSTDC1 in influencing early fracture healing, potentially through the activation of both BMP2 and Wnt signaling pathways.
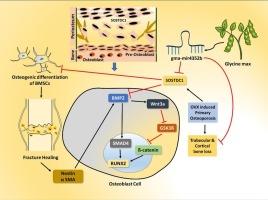
miR4352b 是 SOSTDC1 的跨物种调制剂,以调节骨骼健康和骨折愈合的双重途径为目标。
SOST 基因突变可导致各种单基因骨病。它的同系物 SOSTDC1 蛋白序列同源性为 55%,属于 BMP 拮抗剂类。Sostdc1-/- 小鼠对皮质骨和骨小梁有不同的影响。影响峰值骨量的 SOSTDC1 基因多态性使 SOSTDC1 基因成为影响人类 BMD 变化的候选基因。在骨质流失的情况下,SOSTDC1 会上调,改变 BMP 响应基因和信号调节器,这表明其 BMP/Wnt 双重拮抗剂作用可能会增强这两种途径。过表达 SOSTDC1 证实了其作为成骨拮抗剂的作用。Glycine max(大豆)衍生的 miR4352b 被鉴定为跨领域应用,可精确靶向骨骼的关键调节因子 SOSTDC1。SOSTDC1 与 BMP2 受体 BMPR1A 竞争性结合。Gma-miR4352b 可抑制 SOSTDC1 的表达,从而促进成骨并对抗 SOSTDC1 对成骨潜能的抑制。通过模拟雌激素缺乏导致的 SOSTDC1 水平升高,我们观察到 SOSTDC1 的表达与血清 BMP2 和 PINP 水平呈反相关,而补充 gma-miR4352b 后,血清 BMP2 和 PINP 水平升高。在骨折愈合过程中,SOSTDC1 的关键作用在骨折延迟愈合的情况下显而易见。随着愈合的进展,SOSTDC1 的表达会减少。与加扰 miRNA 相比,Gma-miR4352b 能显著促进胼胝体的形成,到第 10 天,胼胝体愈合率达到 68%,超过加扰组的 44%。到第 13 天,治疗组的愈合速度加快,很难找到胼胝,而加扰组的愈合速度与第 10 天相似。治疗组的加速愈合强调了 SOSTDC1 在影响骨折早期愈合方面的重要性,它可能是通过激活 BMP2 和 Wnt 信号通路来实现的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


