Phylogeny and evolution of dissimilatory sulfite reduction in prokaryotes
IF 3.6
1区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Sulfate is the second most common nonmetallic ion in modern oceans, as its concentration dramatically increased alongside tectonic activity and atmospheric oxidation in the Proterozoic. Microbial sulfate/sulfite metabolism, involving organic carbon or hydrogen oxidation, is linked to sulfur and carbon biogeochemical cycles. However, the coevolution of microbial sulfate/sulfite metabolism and Earth’s history remains unclear. Here, we conducted a comprehensive phylogenetic analysis to explore the evolutionary history of the dissimilatory sulfite reduction (Dsr) pathway. The phylogenies of the Dsr-related genes presented similar branching patterns but also some incongruencies, indicating the complex origin and evolution of Dsr. Among these genes, dsrAB is the hallmark of sulfur-metabolizing prokaryotes. Our detailed analyses suggested that the evolution of dsrAB was shaped by vertical inheritance and multiple horizontal gene transfer events and that selection pressure varied across distinct lineages. Dated phylogenetic trees indicated that key evolutionary events of dissimilatory sulfur-metabolizing prokaryotes were related to the Great Oxygenation Event (2.4–2.0 Ga) and several geological events in the “Boring Billion” (1.8–0.8 Ga), including the fragmentation of the Columbia supercontinent (approximately 1.6 Ga), the rapid increase in marine sulfate (1.3–1.2 Ga), and the Neoproterozoic glaciation event (approximately 1.0 Ga). We also proposed that the voluminous iron formations (approximately 1.88 Ga) might have induced the metabolic innovation of iron reduction. In summary, our study provides new insights into Dsr evolution and a systematic view of the coevolution of dissimilatory sulfur-metabolizing prokaryotes and the Earth’s environment.
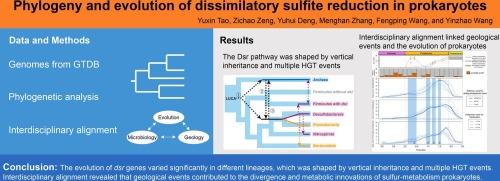
原核生物中异氨亚硫酸盐还原的系统发育和进化。
硫酸盐是现代海洋中第二常见的非金属离子,因为在新生代,随着构造活动和大气氧化,硫酸盐的浓度急剧增加。涉及有机碳或氢氧化的微生物硫酸盐/亚硫酸盐代谢与硫和碳的生物地球化学循环有关。然而,微生物硫酸盐/亚硫酸盐代谢与地球历史的共同演化仍不清楚。在此,我们进行了全面的系统发育分析,以探索亚硫酸盐还原(Dsr)途径的进化历史。Dsr相关基因的系统发育呈现出相似的分支模式,但也存在一些不一致的地方,表明Dsr的起源和进化十分复杂。在这些基因中,dsrAB是硫代谢原核生物的标志。我们的详细分析表明,dsrAB 的进化是由垂直遗传和多个水平基因转移事件决定的,不同品系的选择压力也各不相同。有年代的系统发生树表明,异嗜硫代谢原核生物的关键进化事件与大富氧作用事件(2.4-2.0 Ga)和 "Boring Billion"(1.8-0.8 Ga)中的几个地质事件有关,包括哥伦比亚超大陆的破碎(约 1.6 Ga)、海洋硫酸盐的迅速增加(1.3-1.2 Ga)和新元古代冰川事件(约 1.0 Ga)。我们还提出,大量铁形成(约 1.88 Ga)可能诱发了铁还原的新陈代谢创新。总之,我们的研究为 Dsr 的演化提供了新的视角,并系统地揭示了异嗜硫代谢原核生物与地球环境的共同演化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.50
自引率
7.30%
发文量
249
审稿时长
7.5 months
期刊介绍:
Molecular Phylogenetics and Evolution is dedicated to bringing Darwin''s dream within grasp - to "have fairly true genealogical trees of each great kingdom of Nature." The journal provides a forum for molecular studies that advance our understanding of phylogeny and evolution, further the development of phylogenetically more accurate taxonomic classifications, and ultimately bring a unified classification for all the ramifying lines of life. Phylogeographic studies will be considered for publication if they offer EXCEPTIONAL theoretical or empirical advances.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


