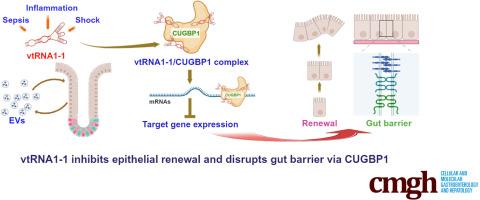
Noncoding Vault RNA1-1 Impairs Intestinal Epithelial Renewal and Barrier Function by Interacting With CUG-binding Protein 1
IF 7.1
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
Cellular and Molecular Gastroenterology and Hepatology
Pub Date : 2024-09-28
DOI:10.1016/j.jcmgh.2024.101410
引用次数: 0
Abstract
Background & Aims
Small noncoding vault RNAs (vtRNAs) are involved in many cell processes important for health and disease, but their pathobiological functions in the intestinal epithelium are underexplored. Here, we investigated the role of human vtRNA1-1 in regulating intestinal epithelial renewal and barrier function.
Methods
Studies were conducted in vtRNA1-1 transgenic (vtRNA1-1Tg) mice, primary enterocytes, and Caco-2 cells. Extracellular vesicles (EVs) were isolated from the serum of shock patients and septic mice. Intestinal organoids (enteroids) were prepared from vtRNA1-1Tg and littermate mice. Mucosal growth was measured by Ki67 immunostaining or BrdU incorporation, and gut permeability was assessed using the FITC-dextran assay.
Results
Intestinal tissues recovered from shock patients and septic mice evidenced mucosal injury and gut barrier dysfunction; vtRNA levels were elevated in EVs isolated from their sera. In mice, intestinal epithelial-specific transgenic expression of vtRNA1-1 inhibited mucosal growth, reduced Paneth cell numbers and intercellular junction (IJ) protein expression, and increased gut barrier vulnerability to lipopolysaccharide exposure. Conversely, in vitro silencing of vtRNA1-1 increased IJ protein levels and enhanced epithelial barrier function. Exposing enteroids to vtRNA1-1-rich EVs augmented paracellular permeability. Mechanistically, vtRNA1-1 interacted with CUG-binding protein 1 (CUGBP1) and increased CUGBP1 association with claudin-1 and occludin mRNAs, thereby inhibiting their expression.
Conclusions
These findings indicate that elevated levels of vtRNA1-1 in EVs and mucosal tissues repress intestinal epithelial renewal and barrier function. Notably, this work reveals a novel role for dysregulation of the vtRNA1-1/CUGBP1 axis in the pathogenesis of gut mucosal disruption in critical illness.
非编码穹隆 RNA1-1 通过与 CUG 结合蛋白 1 相互作用影响肠上皮更新和屏障功能
背景与目的:小非编码穹隆RNA(vtRNA)参与了许多对健康和疾病都很重要的细胞过程,但它们在肠上皮细胞中的病理生物学功能还未得到充分探索。在此,我们研究了人类 vtRNA1-1 在调节肠上皮更新和屏障功能中的作用:研究在 vtRNA1-1 转基因(vtRNA1-1Tg)小鼠、原代肠细胞和 Caco-2 细胞中进行。从休克患者和脓毒症小鼠的血清中分离出细胞外囊泡 (EV)。从 vtRNA1-1Tg 小鼠和同种小鼠中制备了肠组织(enteroids)。通过 Ki67 免疫染色或 BrdU 结合测量粘膜生长,并使用 FITC-葡聚糖检测法评估肠道通透性:结果:从休克患者和败血症小鼠身上提取的肠道组织显示出粘膜损伤和肠道屏障功能障碍;从其血清中分离出的EVs中vtRNA水平升高。在小鼠体内,肠上皮特异性转基因表达 vtRNA1-1 可抑制粘膜生长,减少 Paneth 细胞数量和细胞间连接(IJ)蛋白表达,并增加肠道屏障对脂多糖暴露的脆弱性。相反,体外沉默 vtRNA1-1 可提高 IJ 蛋白水平,增强上皮屏障功能。将肠道暴露于富含vtRNA1-1的EV会增加细胞旁通透性。从机制上讲,vtRNA1-1与CUG结合蛋白1(CUGBP1)相互作用,增加了CUGBP1与claudin-1和occludin mRNA的结合,从而抑制了它们的表达:这些研究结果表明,EVs 和粘膜组织中 vtRNA1-1 水平的升高抑制了肠上皮的更新和屏障功能。值得注意的是,这项研究揭示了 vtRNA1-1/CUGBP1 轴失调在危重症肠道粘膜损伤发病机制中的新作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular and Molecular Gastroenterology and Hepatology
Medicine-Gastroenterology
CiteScore
13.00
自引率
2.80%
发文量
246
审稿时长
42 days
期刊介绍:
"Cell and Molecular Gastroenterology and Hepatology (CMGH)" is a journal dedicated to advancing the understanding of digestive biology through impactful research that spans the spectrum of normal gastrointestinal, hepatic, and pancreatic functions, as well as their pathologies. The journal's mission is to publish high-quality, hypothesis-driven studies that offer mechanistic novelty and are methodologically robust, covering a wide range of themes in gastroenterology, hepatology, and pancreatology.
CMGH reports on the latest scientific advances in cell biology, immunology, physiology, microbiology, genetics, and neurobiology related to gastrointestinal, hepatobiliary, and pancreatic health and disease. The research published in CMGH is designed to address significant questions in the field, utilizing a variety of experimental approaches, including in vitro models, patient-derived tissues or cells, and animal models. This multifaceted approach enables the journal to contribute to both fundamental discoveries and their translation into clinical applications, ultimately aiming to improve patient care and treatment outcomes in digestive health.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


