Intradermal and transdermal absorption of beta-naphthylamine and N-Phenyl-beta-naphthylamine in a viable human skin model
IF 2.7
3区 医学
Q3 TOXICOLOGY
引用次数: 0
Abstract
Technical products containing N-Phenyl-beta-naphthylamine (PBNA) are contaminated with beta-naphthylamine (BNA), a known carcinogen. Both amines penetrate the skin to different degrees, but little is known about their dermal-depot formation. This study investigated the dermal penetration of PBNA and its degradation product BNA using a viable human-skin model. PBNA (259 μg) or BNA (0.52 μg) in n-hexane and industrial grease were applied to freshly excised human skin (n = 6, 0.64 cm2) for 2-72 h. After temporary/continuous and single/repeated exposure, samples were taken (stratum corneum, epidermis/dermis, receptor fluid) and analyzed for their amine content by GC–MS. Continuous exposure led to a PBNA dermal depot of ∼47 μg/cm2 over 72 h. Temporary applications also resulted in lower but consistent PBNA dermal depots. A single 2-h application resulted in a dermal depot of ∼16 μg/cm2 after 72 h, while this was ∼25 μg/0.64 cm2 with repeated applications. BNA behaved differently; with repeated 2-h applications, intradermally retained BNA initially increased 3–6 fold, then dropped to ∼200–250 ng/cm2. This incomplete decline upon repeated short-term exposure to PBNA suggests that a BNA dermal depot is formed either due to contamination of PBNA with BNA or to enzymatic conversion of PBNA to BNA. Additionally, PBNA dermal depots were saturable under the given conditions. These findings highlight the importance of understanding the dermal-exposure dynamics of potential carcinogenic compounds in industrial settings.
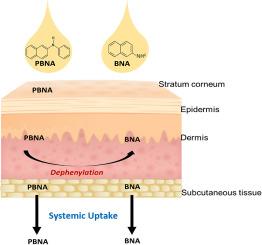
活体人体皮肤模型对 beta-萘胺和 n-苯基-beta-萘胺的皮内吸收和透皮吸收。
含有正苯基-beta-萘胺(PBNA)的技术产品受到已知致癌物质--beta-萘胺(BNA)的污染。这两种胺都能不同程度地渗透皮肤,但人们对它们的皮肤沉积形成知之甚少。本研究使用一个有活力的人体皮肤模型对 PBNA 及其降解产物 BNA 的皮肤渗透性进行了研究。将正己烷和工业油脂中的 PBNA(259 μg)或 BNA(0.52 μg)涂抹在新鲜切除的人体皮肤(n = 6,0.64 平方厘米)上 2-72 小时。在临时/连续和单次/重复接触后,采集样本(角质层、表皮/真皮、受体液)并通过气相色谱-质谱分析其胺含量。连续接触 72 小时后,PBNA 在皮肤中的含量约为 47 微克/平方厘米。临时施用也会导致较低但一致的 PBNA 皮肤沉积。单次施用 2 小时后,72 小时后的真皮沉积物约为 16 微克/平方厘米,而重复施用时的真皮沉积物约为 25 微克/0.64 平方厘米。BNA 的表现不同;重复施用 2 小时后,皮内保留的 BNA 最初增加了 3-6 倍,然后降至约 200-250 ng/cm2。反复短期暴露于 PBNA 后的这种不完全下降表明,由于 PBNA 与 BNA 的污染或 PBNA 向 BNA 的酶转化,形成了 BNA 皮肤库。此外,在特定条件下,PBNA 皮肤库是饱和的。这些发现凸显了了解工业环境中潜在致癌化合物的皮肤暴露动态的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicology in Vitro
医学-毒理学
CiteScore
6.50
自引率
3.10%
发文量
181
审稿时长
65 days
期刊介绍:
Toxicology in Vitro publishes original research papers and reviews on the application and use of in vitro systems for assessing or predicting the toxic effects of chemicals and elucidating their mechanisms of action. These in vitro techniques include utilizing cell or tissue cultures, isolated cells, tissue slices, subcellular fractions, transgenic cell cultures, and cells from transgenic organisms, as well as in silico modelling. The Journal will focus on investigations that involve the development and validation of new in vitro methods, e.g. for prediction of toxic effects based on traditional and in silico modelling; on the use of methods in high-throughput toxicology and pharmacology; elucidation of mechanisms of toxic action; the application of genomics, transcriptomics and proteomics in toxicology, as well as on comparative studies that characterise the relationship between in vitro and in vivo findings. The Journal strongly encourages the submission of manuscripts that focus on the development of in vitro methods, their practical applications and regulatory use (e.g. in the areas of food components cosmetics, pharmaceuticals, pesticides, and industrial chemicals). Toxicology in Vitro discourages papers that record reporting on toxicological effects from materials, such as plant extracts or herbal medicines, that have not been chemically characterized.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


