Evaluation of a New In Chemico Skin Corrosion Test
IF 2.6
3区 医学
Q3 TOXICOLOGY
引用次数: 0
Abstract
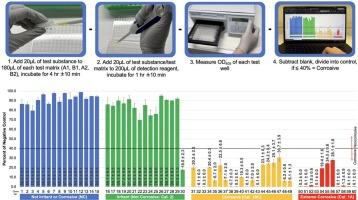
The purpose of this study is to evaluate a novel macromolecular test method for the identification of dermal corrosives. The simple in chemico test procedure involves allowing the material to be tested to interact with a skin biomarker for corrosivity and then adding a detection reagent. The corrosivity of the test substance is predicted based on the measured macromolecular damage, which results in reduced optical density of the detection reagent as compared with controls. This study aims to determine if such an extremely simple, cell-free test method can accurately identify dermal corrosives. To determine predictivity and repeatability, we tested 60 chemicals (30 in vivo dermal corrosives and 30 in vivo dermal noncorrosives; all tested in triplicate) representative of a broad range of chemical classes, functional groups, mixtures, and levels of toxicity. Validation results indicate the GHS multicategory and packing group assignment accuracy is on par with that of the Reconstructed Human Epidermis test method and the Membrane Barrier test method and for the global identification of corrosives, the method has a considerably higher accuracy (98 % vs. ∼80 %).
评估新的皮肤腐蚀化学测试。
本研究的目的是评估一种新型的大分子测试方法,用于鉴定皮肤腐蚀剂。简单的化学测试程序包括让待测物质与皮肤生物标志物发生腐蚀性作用,然后加入检测试剂。测试物质的腐蚀性是根据测得的大分子损伤来预测的,与对照组相比,大分子损伤会导致检测试剂的光密度降低。本研究旨在确定这种极其简单的无细胞测试方法能否准确识别皮肤腐蚀剂。为了确定预测性和可重复性,我们测试了 60 种化学物质(30 种体内皮肤腐蚀剂和 30 种体内皮肤非腐蚀剂;所有测试均一式三份),这些化学物质代表了广泛的化学类别、功能群、混合物和毒性水平。验证结果表明,全球统一制度多类别和包装组分配的准确性与重建人体表皮测试法和膜阻隔性测试法相当,而在腐蚀剂的全面鉴定方面,该方法的准确性要高得多(98% 对 ~80%)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicology in Vitro
医学-毒理学
CiteScore
6.50
自引率
3.10%
发文量
181
审稿时长
65 days
期刊介绍:
Toxicology in Vitro publishes original research papers and reviews on the application and use of in vitro systems for assessing or predicting the toxic effects of chemicals and elucidating their mechanisms of action. These in vitro techniques include utilizing cell or tissue cultures, isolated cells, tissue slices, subcellular fractions, transgenic cell cultures, and cells from transgenic organisms, as well as in silico modelling. The Journal will focus on investigations that involve the development and validation of new in vitro methods, e.g. for prediction of toxic effects based on traditional and in silico modelling; on the use of methods in high-throughput toxicology and pharmacology; elucidation of mechanisms of toxic action; the application of genomics, transcriptomics and proteomics in toxicology, as well as on comparative studies that characterise the relationship between in vitro and in vivo findings. The Journal strongly encourages the submission of manuscripts that focus on the development of in vitro methods, their practical applications and regulatory use (e.g. in the areas of food components cosmetics, pharmaceuticals, pesticides, and industrial chemicals). Toxicology in Vitro discourages papers that record reporting on toxicological effects from materials, such as plant extracts or herbal medicines, that have not been chemically characterized.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


