Managing the unmanageable: evidence-driven approaches to real-world patient prototypes of TP53-mutant myelodysplastic neoplasms and acute myeloid leukemia
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
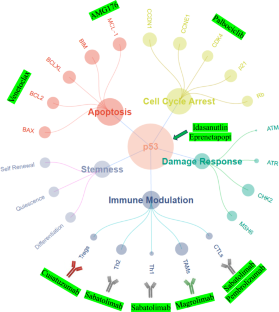
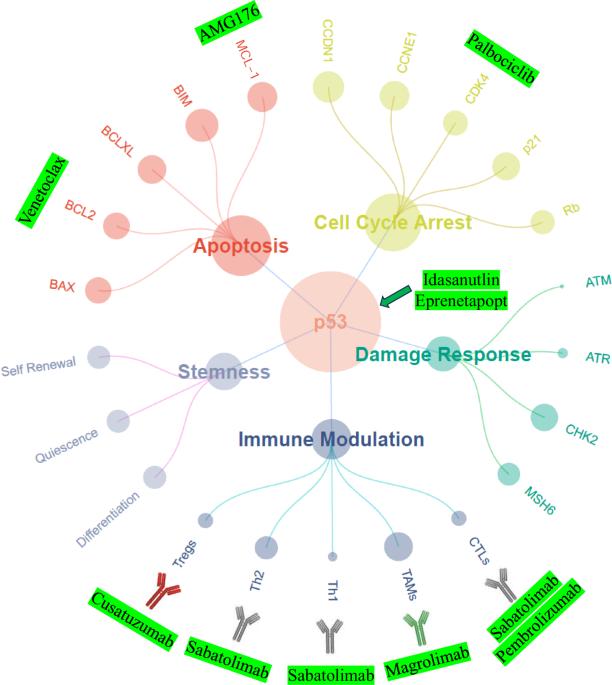
Patients with TP53 aberrations comprise the highest risk subset of all myeloid malignancies. The managerial conundrum of TP53-mutant myelodysplastic neoplasms (MDS) and acute myeloid leukemia (AML) stems from refractoriness to or relapse after conventional chemotherapy, as well as the limited translational success of investigational therapies targeting TP53-mutant cells. Thus far, no targeted therapies have been commercially approved for this mutational subset. As a result, management plans for patients with TP53-mutant MDS and AML are often driven by clinical judgment and/or physician preference rather than consensus guidelines backed by a rigorous evidence basis. This clinical case-based, evidence-driven review highlights the most salient data that guides the management of commonly encountered patient prototypes. This review discusses the therapeutic menu of first-line options that derive from multi-institutional experiences as well as from disease-centric consortia and discusses how these first-line options can be optimally tailored to heterogeneous groups of patients. The debate regarding whether allogeneic stem cell transplant should be offered to these patients is summarized. Finally, this review explores the recent unfortunate news of pauses in clinical trials for the leading investigational agents – eprenetapopt, magrolimab, sabatolimab, and idasanutlin – and offers solutions toward re-invigorating the pipeline of precision therapeutics in 2025.
处理无法处理的问题:针对真实世界中 TP53 突变骨髓增生异常肿瘤和急性髓性白血病患者原型的循证方法
TP53 基因畸变患者是所有骨髓恶性肿瘤中风险最高的亚群。TP53突变骨髓增生异常性肿瘤(MDS)和急性髓性白血病(AML)的管理难题源于传统化疗后的难治性或复发,以及针对TP53突变细胞的研究性疗法的转化成功率有限。迄今为止,还没有针对这一突变亚群的靶向疗法获得商业批准。因此,TP53 突变 MDS 和 AML 患者的治疗计划往往是由临床判断和/或医生偏好驱动的,而不是以严格的证据为基础的共识指南。这篇以临床病例为基础、以证据为导向的综述强调了指导常见患者原型管理的最重要数据。本综述讨论了从多机构经验和以疾病为中心的联盟中得出的一线治疗方案菜单,并讨论了如何针对不同患者群体优化这些一线方案。本综述还总结了有关是否应为这些患者提供异基因干细胞移植的争论。最后,这篇综述探讨了最近关于主要研究药物--依普瑞奈普特、马格列单抗、沙巴妥利单抗和依达沙奴特林--临床试验暂停的不幸消息,并提出了在2025年重新激活精准治疗管线的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


