Decoding the Promotional Effect of Iron in Bimetallic Pt–Fe-nanoparticles on the Low Temperature Reverse Water–Gas Shift Reaction
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
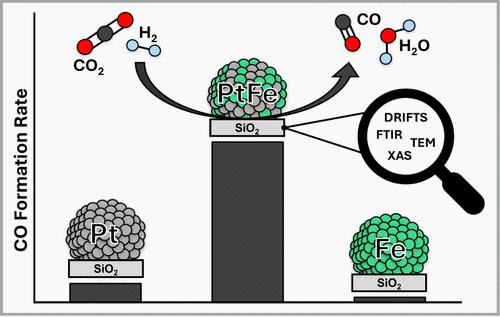
The reverse water–gas shift (RWGS) reaction is a key technology of the chemical industry, central to the emerging circular carbon economy. Pt-based catalysts have previously been shown to effectively promote RWGS, especially when modified by promoter elements. However, their active states are still poorly understood. Here, we show that the intimate incorporation of an iron promoter into metal-oxide-supported Pt-based nanoparticles can increase their activity and selectivity for the low temperature reverse water–gas shift (LT-RWGS) substantially and drastically outperform unpromoted Pt-based materials. Specifically, the study explores the promotional effect of iron in Pt–Fe bimetallic systems supported on silica (PtxFey@SiO2) prepared by surface organometallic chemistry (SOMC). The most active catalyst (Pt1Fe1@SiO2) shows high selectivity (>99% CO) toward CO at a formation rate of 0.192 molCO h–1 gcat–1, which is significantly higher than that of monometallic Pt@SiO2 (96% sel. and 0.022 molCO h–1 gcat–1). In-situ diffuse reflectance FT-IR spectroscopy (DRIFTS) and X-ray absorption spectroscopy (XAS) indicate a dynamic process at the catalyst surface under the reaction conditions, revealing distinct reaction pathways for the monometallic Pt@SiO2 and bimetallic PtxFey@SiO2 systems.
解码双金属铂-铁-纳米粒子中的铁对低温反向水-气变换反应的促进作用
水煤气反向转化(RWGS)反应是化学工业的一项关键技术,也是新兴循环碳经济的核心。此前已有研究表明,铂基催化剂能有效促进 RWGS 反应,尤其是在经过促进剂元素修饰的情况下。然而,人们对它们的活性状态仍然知之甚少。在这里,我们展示了在金属氧化物支撑的铂基纳米粒子中紧密加入铁促进剂,可大幅提高其在低温水气反向变换(LT-RWGS)中的活性和选择性,并大大优于未加入促进剂的铂基材料。具体而言,本研究探讨了通过表面有机金属化学(SOMC)制备的、支撑在二氧化硅(PtxFey@SiO2)上的铂铁双金属体系中铁的促进作用。活性最高的催化剂(Pt1Fe1@SiO2)对一氧化碳具有高选择性(99% CO),其生成率为 0.192 molCO h-1 gcat-1,明显高于单金属 Pt@SiO2(96% sel.和 0.022 molCO h-1 gcat-1)。原位漫反射傅立叶变换红外光谱(DRIFTS)和 X 射线吸收光谱(XAS)表明,在反应条件下催化剂表面存在一个动态过程,揭示了单金属 Pt@SiO2 和双金属 PtxFey@SiO2 系统的不同反应途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


