Reactive Passivation of Wide-Bandgap Organic–Inorganic Perovskites with Benzylamine
IF 3.784
3区 化学
Q1 Chemistry
引用次数: 0
Abstract
While amines are widely used as additives in metal-halide perovskites, our understanding of the way amines in perovskite precursor solutions impact the resultant perovskite film is still limited. In this paper, we explore the multiple effects of benzylamine (BnAm), also referred to as phenylmethylamine, used to passivate both FA0.75Cs0.25Pb(I0.8Br0.2)3 and FA0.8Cs0.2PbI3 perovskite compositions. We show that, unlike benzylammonium (BnA+) halide salts, BnAm reacts rapidly with the formamidinium (FA+) cation, forming new chemical products in solution and these products passivate the perovskite crystal domains when processed into a thin film. In addition, when BnAm is used as a bulk additive, the average perovskite solar cell maximum power point tracked efficiency (for 30 s) increased to 19.3% compared to the control devices 16.8% for a 1.68 eV perovskite. Under combined full spectrum simulated sunlight and 65 °C temperature, the devices maintained a better T80 stability of close to 2500 h while the control devices have T80 stabilities of <100 h. We obtained similar results when presynthesizing the product BnFAI and adding it directly into the perovskite precursor solution. These findings highlight the mechanistic differences between amine and ammonium salt passivation, enabling the rational design of molecular strategies to improve the material quality and device performance of metal-halide perovskites.
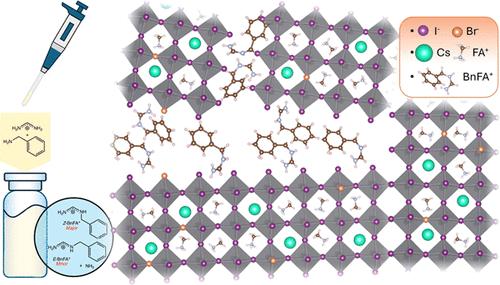
用苄胺反应钝化宽带隙有机无机包光体
虽然胺被广泛用作金属卤化物包晶石的添加剂,但我们对包晶石前驱体溶液中的胺如何影响所产生的包晶石薄膜的了解仍然有限。在本文中,我们探讨了用于钝化 FA0.75Cs0.25Pb(I0.8Br0.2)3 和 FA0.8Cs0.2PbI3 包晶成分的苄胺(BnAm)(也称为苯基甲胺)的多重影响。我们的研究表明,与苄基铵(BnA+)卤化物盐不同,BnAm 会迅速与甲脒阳离子(FA+)发生反应,在溶液中形成新的化学产物,这些产物在加工成薄膜时会钝化包晶晶体畴。此外,当使用 BnAm 作为块状添加剂时,与 1.68 eV 包晶的对照设备 16.8% 的效率相比,包晶太阳能电池的平均最大功率点跟踪效率(30 秒)提高到了 19.3%。在全光谱模拟太阳光和 65 °C 温度条件下,器件的 T80 稳定性更好,接近 2500 小时,而对照器件的 T80 稳定性为 100 小时。这些发现凸显了胺盐和铵盐钝化之间的机理差异,有助于合理设计分子策略,提高金属卤化物包晶材料的质量和器件性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Combinatorial Science
CHEMISTRY, APPLIED-CHEMISTRY, MEDICINAL
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
The Journal of Combinatorial Chemistry has been relaunched as ACS Combinatorial Science under the leadership of new Editor-in-Chief M.G. Finn of The Scripps Research Institute. The journal features an expanded scope and will build upon the legacy of the Journal of Combinatorial Chemistry, a highly cited leader in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


