Multistep screening of transition-metal-based homonuclear double-atom catalysts to unravel the electronic origin of their activity and selectivity challenges for nitrogen reduction†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Lack of robust catalyst design strategies for tackling the selectivity and activity challenges poses serious limitations in the development of efficient catalysts for nitrogen reduction to ammonia. The synergistic interactions in double-atom catalysts (DACs) have aroused great interest in developing promising catalytic centers for the nitrogen reduction reaction (NRR). Using a multistep screening strategy based on systematic first-principles simulations, we find that Fe2, Co2, and W2 dimer species impregnated in a tetracyanoquinodimethane-based monolayer achieve suitable adsorption behaviour for the various NRR intermediates, leading to excellent activity and selectivity among the 27 DACs considered in this study for the NRR. Interestingly, our results reveal very low limiting potential values of −0.56, −0.58, and −0.53 V for Fe2, Co2, and W2, respectively, compared to the experimentally reported values of −0.73 and −0.98 V for the Ru-based single-atom catalyst and Ru(0001) stepped surface. Density of states analysis indicated that the adsorption pattern of the reaction intermediates was regulated by the d-states of the DACs near the Fermi level. Correlation trends between the limiting potential and the free energy change for adsorption of different intermediates show that the free energy change for N2 adsorption proves a suitable guidance to evaluate the NRR activity of the modelled catalysts. Further, rigorous electronic structure analysis highlighted properties such as integrated crystal orbital Hamilton populations and orbital projected density of states, and the d-band centre could be successfully used to rationalize the N2 binding and adsorption on these catalysts. Thus, this work provides a feasible design strategy for NRR electrocatalysis based on extensive electronic structure concepts.
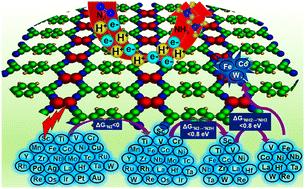
多步筛选过渡金属基同核双原子催化剂,揭示其氮还原活性和选择性挑战的电子起源†。
缺乏稳健的催化剂设计策略来应对选择性和活性方面的挑战,严重限制了氮还原为氨的高效催化剂的开发。双原子催化剂 (DAC) 中的协同作用引起了人们对开发氮还原反应 (NRR) 催化中心的极大兴趣。利用基于系统第一性原理模拟的多步筛选策略,我们发现浸渍在基于四氰基二甲烷的单层中的 Fe2、Co2 和 W2 二聚体对各种氮还原反应中间产物具有合适的吸附行为,从而在本研究中考虑的 27 种用于氮还原反应的双原子催化剂中获得了优异的活性和选择性。有趣的是,我们的研究结果显示,Fe2、Co2 和 W2 的极限电势值分别为 -0.56、-0.58 和 -0.53V,而实验报告中 Ru 基单原子催化剂和 Ru(0001) 阶梯表面的极限电势值分别为 -0.73 和 -0.98V。状态密度分析表明,反应中间产物的吸附模式受费米级附近 DAC 的 d 态调节。极限电势与吸附不同中间产物的自由能变化之间的相关趋势表明,吸附 N2 的自由能变化证明是评估模型催化剂 NRR 活性的合适指导。此外,严格的电子结构分析突出显示了集成晶体轨道哈密尔顿种群和轨道投影状态密度以及 d 带中心等特性,可成功用于合理解释催化剂上的 N2 结合和吸附。因此,这项工作为基于广泛电子结构概念的 NRR 电催化提供了可行的设计策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


