Highly efficient cobalt catalysts promoted by CeO2–Al2O3 for ammonia decomposition†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
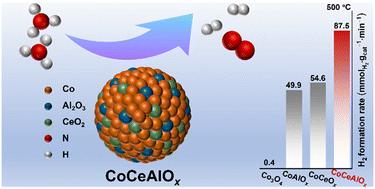
Hydrogen production by ammonia decomposition reveals great advantages in the utilization of hydrogen. Low-cost, efficient and stable transition metal catalysts are the key for the ammonia decomposition reaction. Using multi-component promoters adjust the electronic and geometric structures of bulk catalysts, which might be an effective approach for obtaining non-noble metal catalysts with excellent performance. In this work, CeO2–Al2O3 bi-promoters were used and significantly improved the catalytic performance of bulk cobalt catalysts. The optimized CoCeAlOx catalyst achieved 94.3% and 65.3% NH3 conversion at 550 °C and 500 °C (GHSV = 30 000 mL gcat−1 h−1), respectively, and exhibited strong stability within 200 h. It was found that the interaction between Co–CeO2–Al2O3 effectively inhibited the aggregation of the Co0 active species, which enormously promoted the catalytic activity and stability of CoCeAlOx. CeO2–Al2O3 bi-promoters adjusted the surface properties of catalysts, bringing suitable NH3 adsorption and N2 desorption, and suppressing the hydrogen poisoning significantly. This work provided a reliable strategy for the construction of high-efficiency catalysts working under the harsh conditions for ammonia decomposition.
CeO2-Al2O3 促进的高效钴催化剂用于氨分解†.
氨分解制氢在氢气利用方面具有巨大优势。低成本、高效和稳定的过渡金属催化剂是氨分解反应的关键。利用多组分促进剂调整块体催化剂的电子和几何结构,可能是获得性能优异的非贵金属催化剂的有效方法。在这项工作中,使用了 CeO2-Al2O3 双促进剂,显著提高了块状钴催化剂的催化性能。研究发现,Co-CeO2-Al2O3 之间的相互作用有效抑制了 Co0 活性物种的聚集,极大地提高了 CoCeAlOx 的催化活性和稳定性。CeO2-Al2O3 双促进剂调整了催化剂的表面性质,带来了合适的 NH3 吸附和 N2 解吸,显著抑制了氢中毒。这项工作为在苛刻的氨分解条件下构建高效催化剂提供了可靠的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


