Co(iii) corroles with pendant amidophenol and amidopyridine as proton-relay arms to facilitate the electrocatalytic hydrogen evolution reaction†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
It is known that the efficiency of the hydrogen evolution reaction (HER) can be promoted by the presence of proton relay groups neighboring a catalytic metal center. This work reports the synthesis of two new Co(iii) corroles (2 and 3) with hangman proton relay groups for the electrocatalytic HER. When using trifluoroacetic acid (TFA) as a proton source, complexes 2 and 3 exhibited turnover frequencies (TOFs) of 219.36 s−1 and 199.57 s−1, respectively. The catalytic HER undergoes via the EECEC or EECC pathway (E: electron transfer; C: charge transfer) depending on the acidity and concentration of the proton source. Moreover, complex 2 and 3 also showed good HER activity in aqueous media with Faraday efficiencies (FEs) of 93.6% and 89.2% respectively.
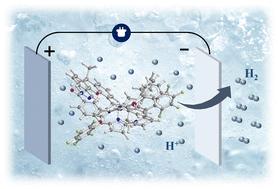
Co(iii)腐蚀剂以悬挂的氨基苯酚和氨基吡啶作为质子中继臂,促进电催化氢进化反应†。
众所周知,催化金属中心邻近质子中继基团的存在可提高氢进化反应(HER)的效率。本研究报告合成了两种带有质子中继基团的新型 Co(III) 腐蚀物(2 和 3),用于电催化氢进化反应。当使用三氟乙酸(TFA)作为质子源时,配合物 2 和 3 的翻转频率(TOFs)分别为 219.36 s-1 和 199.57 s-1。根据质子源的酸度和浓度,催化 HER 经过 EECEC 或 EECC 途径(E:电子转移;C:电荷转移)。此外,复合物 2 和 3 在水介质中也表现出良好的 HER 活性,法拉第效率(FE)分别为 93.6% 和 89.2%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


