BCl3 catalyzed Z-selective intramolecular chlorocarbamoylation of alkynes/allenes†
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A metal-free stereoselective intramolecular chlorocarbamoylation of alkynes/allenes driven by BCl3 was described, which provides a general and practical method for constructing versatile and chlorinated methylene lactams or tetrahydroisoquinolinones with (Z) geometry at the double bond, featuring a stereochemically defined tetrasubstituted chloroethene.
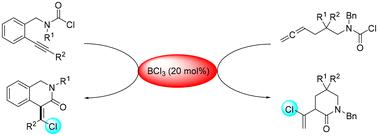
BCl3 催化炔/烯的 Z 选择性分子内氯氨基甲酰化反应
该研究描述了一种在 BCl3 驱动下对炔/烯进行无金属立体选择性分子内氯氨基甲酰化的方法,它为构建双键处具有 (Z) 几何结构的多功能氯化亚甲基内酰胺或四氢异喹啉酮提供了一种通用而实用的方法,其特点是具有立体化学定义的四取代氯乙烯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


