Prognostic value of response to first-line hydroxyurea according to IPSET stratification in essential thrombocythemia
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Hydroxyurea (HU) constitutes the first-line treatment in most patients with essential thrombocythemia (ET), but criteria for changing therapy are not clearly established. The prognostic value of complete hematological response (CHR) and resistance/intolerance to HU was assessed in 1080 patients from the Spanish Registry of ET, classified according to revised IPSET-Thrombosis stratification (Very low- n = 61, Low- n = 83, Intermediate- n = 261, and High-risk n = 675). With a median therapy duration of 5 years, CHR was registered in 720 (67%) patients (1-year probability 51%) and resistance/intolerance in 219 (20%) patients (5-years probability 13%). After correction by other risk factors, High-risk patients achieving CHR showed a reduced risk of arterial thrombosis (HR: 0.35, 95%CI: 0.2–0.6, p = 0.001) and a trend towards lower risk of venous thrombosis (HR: 0.45, 95%CI: 0.2–1.02, p = 0.06) whereas no association was observed for intermediate- or low-risk patients. In comparison with non-responders, intermediate- and high-risk patients achieving CHR had longer survival and lower myelofibrosis incidence. Development of resistance/intolerance to HU, mainly cytopenia, was associated with higher probability of myelofibrosis but no effect on survival or thrombotic risk was demonstrated. In conclusion, CHR with HU is associated with better outcomes and might be an early indicator for selecting candidates to second-line clinical trials.
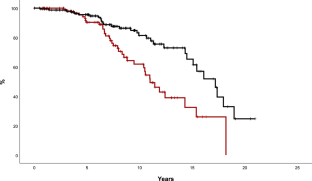
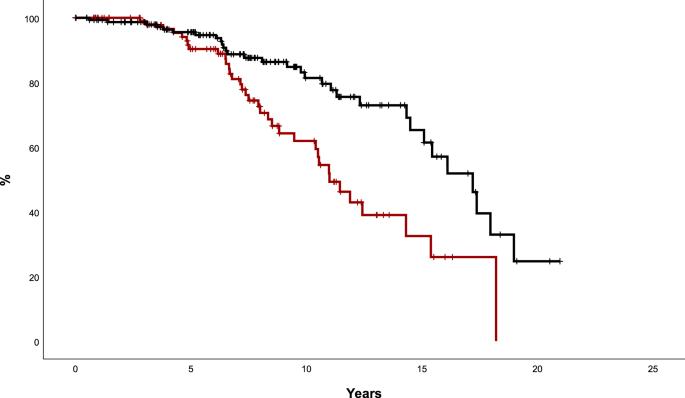
根据 IPSET 分层对原发性血小板增多症患者一线羟基脲反应的预后价值
羟基脲(HU)是大多数原发性血小板增多症(ET)患者的一线治疗方法,但改变疗法的标准尚未明确确立。我们对西班牙 ET 登记处的 1080 名患者的完全血液学反应(CHR)和对 HU 的耐药性/不耐受性的预后价值进行了评估,并根据修订后的 IPSET 血栓分层法进行了分类(极低风险 61 人,低风险 83 人,中度风险 261 人,高风险 675 人)。中位治疗持续时间为 5 年,720 例(67%)患者出现 CHR(1 年概率为 51%),219 例(20%)患者出现耐药/不耐受(5 年概率为 13%)。在对其他风险因素进行校正后,实现 CHR 的高危患者动脉血栓风险降低(HR:0.35,95%CI:0.2-0.6,p = 0.001),静脉血栓风险呈降低趋势(HR:0.45,95%CI:0.2-1.02,p = 0.06),而中危或低危患者则无相关性。与无应答者相比,达到CHR的中危和高危患者生存期更长,骨髓纤维化发生率更低。出现对 HU 的耐药性/不耐受性(主要是细胞减少)与骨髓纤维化的可能性增加有关,但对生存期或血栓风险没有影响。总之,使用 HU 的 CHR 与更好的预后相关,可作为选择二线临床试验候选者的早期指标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


