Characterization of myeloproliferative neoplasms based on genetics only and prognostication of transformation to blast phase
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
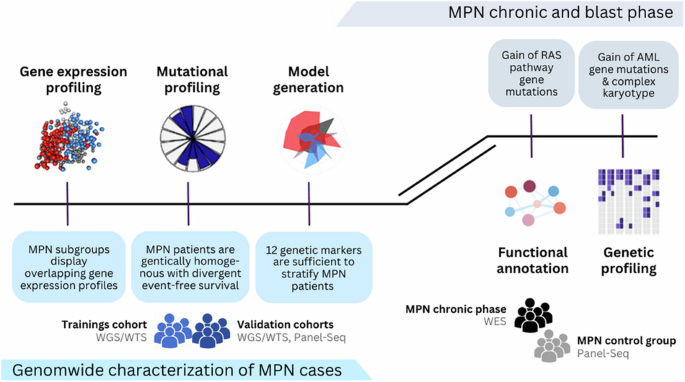
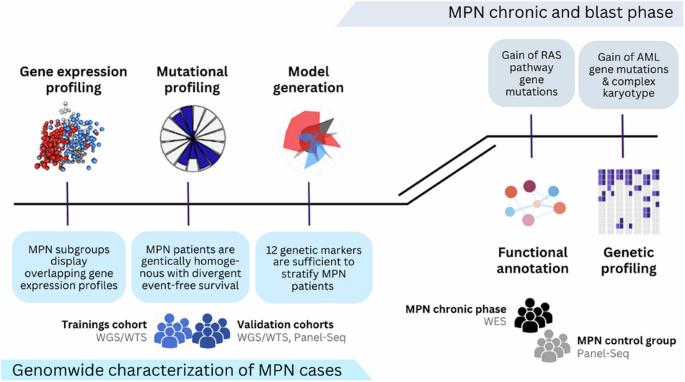
Myeloproliferative neoplasms (MPN) are a heterogeneous group of clonal disorders characterized by aberrant hematopoietic proliferation and an intrinsic risk of progression to blast phase. The WHO classification 2022 identifies chronic myeloid leukemia and the BCR::ABL1 negative MPNs polycythemia vera, primary myelofibrosis and essential thrombocythemia as individual entities. However, overlaps, borderline findings or transitions between MPN subtypes occur and incomplete clinical data often complicates diagnosis. By conducting a thorough genetic analysis, we’ve developed a model that relies on 12 genetic markers to accurately stratify MPN patients. The model can be simplified into a decision tree for routine use. Comparing samples at chronic and blast phase revealed, that one third of patients lost their MPN driver-gene mutation, while mutations in splicing and chromatin modifying genes were stable, indicating a shared founder clone of chronic and blast phase with different driver mutations and therefore different progressing capacities. This was further supported by gain of typical de novo AML gene mutations, accompanied by gain of complex karyotypes and RAS pathway gene mutations. Our data suggest to perform a broader genetic screening at diagnosis and also at clinical progression, as driver mutations may change and the MPN-driver mutations present at diagnosis may disappear.
仅根据遗传学对骨髓增生性肿瘤进行定性,并对向胚泡期转化的预后进行预测
骨髓增殖性肿瘤(MPN)是一组异质性克隆性疾病,其特点是异常造血增殖和固有的发展到爆炸期的风险。世卫组织 2022 年分类将慢性髓性白血病和 BCR::ABL1 阴性 MPNs 多发性红细胞增多症、原发性骨髓纤维化和原发性血小板增多症视为单独的实体。然而,多发性骨髓瘤亚型之间会出现重叠、边界发现或过渡,而且不完整的临床数据往往会使诊断复杂化。通过全面的基因分析,我们建立了一个模型,该模型依靠 12 个基因标记物对 MPN 患者进行准确分层。该模型可简化为决策树,供常规使用。对慢性期和爆发期样本进行比较后发现,三分之一的患者失去了他们的 MPN 驱动基因突变,而剪接基因和染色质修饰基因的突变则保持稳定,这表明慢性期和爆发期的共同创始克隆具有不同的驱动基因突变,因此具有不同的进展能力。典型的新发急性髓细胞性白血病基因突变的增加,以及复杂核型和RAS通路基因突变的增加进一步证实了这一点。我们的数据建议在诊断和临床进展时进行更广泛的基因筛查,因为驱动基因突变可能会发生变化,诊断时存在的 MPN 驱动基因突变可能会消失。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


