Neuronal LAMP2A-mediated reduction of adenylyl cyclases induces acute neurodegenerative responses and neuroinflammation after ischemic stroke
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Lysosomes regulate cellular metabolism to maintain cell survival, but the mechanisms whereby they determine neuronal cell fate after acute metabolic stress are unknown. Neuron-enriched lysosomal membrane protein LAMP2A is involved in selective chaperone-mediated autophagy and exosome loading. This study demonstrates that abnormalities in the neuronal LAMP2A-lysosomal pathway cause neurological deficits following ischemic stroke and that this is an early inducer of the PANoptosis-like molecular pathway and neuroinflammation, simultaneously inducing upregulation of FADD, RIPK3, and MLKL after ischemia. Quantitative proteomic and pharmacological analysis showed that after acute metabolic stress, the neuronal LAMP2A pathway induced acute synaptic degeneration and PANoptosis-like responses involving downregulation of protein kinase A (PKA) signaling. LAMP2A directed post-stroke lysosomal degradation of adenylyl cyclases (ADCY), including ADCY1 and ADCY3 in cortical neurons. Post-stroke treatment with cAMP mimetic or ADCY activator salvaged cortical neurons from PANoptosis-like responses and neuroinflammation, suggesting that the neuronal ADCY–cAMP–PKA axis is an upstream arrester of the pathophysiological process following an ischemic stroke. This study demonstrates that the neuronal LAMP2A-lysosmal pathway drives intricate acute neurodegenerative and neuroinflammatory responses after brain metabolic stress by downregulating the ADCY–PKA signaling cascade, and highlights the therapeutic potential of PKA signal inducers for improving stroke outcomes.

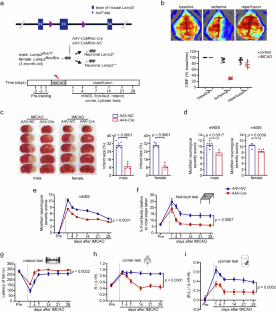
神经元 LAMP2A 介导的腺苷酸环化酶减少诱导缺血性中风后的急性神经退行性反应和神经炎症
溶酶体调节细胞新陈代谢以维持细胞存活,但它们在急性代谢应激后决定神经细胞命运的机制尚不清楚。神经元富集的溶酶体膜蛋白 LAMP2A 参与了选择性伴侣介导的自噬和外泌体装载。本研究表明,神经元LAMP2A-溶酶体通路的异常会导致缺血性脑卒中后的神经功能缺损,而且这是缺血后PAN凋亡样分子通路和神经炎症的早期诱导因素,同时诱导FADD、RIPK3和MLKL的上调。定量蛋白质组学和药理学分析表明,在急性代谢应激后,神经元 LAMP2A 通路诱导急性突触变性和 PANoptosis 样反应,其中涉及蛋白激酶 A(PKA)信号的下调。LAMP2A 引导了中风后大脑皮质神经元中腺苷酸环化酶(ADCY)(包括 ADCY1 和 ADCY3)的溶酶体降解。中风后使用 cAMP 模拟物或 ADCY 激活剂可挽救皮质神经元免受 PANoptosis-like 反应和神经炎症的影响,这表明神经元 ADCY-cAMP-PKA 轴是缺血性中风后病理生理过程的上游阻断器。这项研究表明,神经元 LAMP2A-lysosmal 通路通过下调 ADCY-PKA 信号级联,驱动脑代谢应激后错综复杂的急性神经退行性变和神经炎症反应,并强调了 PKA 信号诱导剂在改善中风预后方面的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
文献相关原料
公司名称
产品信息
阿拉丁
titanium oxide
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


