Revealing the Unusual behavior of rhenium (I) tricarbonyl complex functionalized with Aza-Macrocycles in response to metal ions
IF 2.7
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
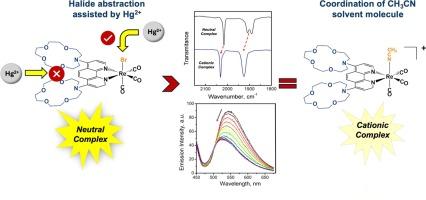
Three new rhenium phenanthroline tricarbonyl complexes were synthesized and fully characterized to examine changes in their photophysical properties and spectroscopic behavior in the presence of metal ions. To achieve this, the complexes were designed with modifications in both the phenanthroline and the axial ligand. The 4,7–dichloro–1,10–phenanthroline (Cl2phen) precursor was functionalized with two units of 1–aza–15–crown–5 (1A15C5) to obtain the complex [Re((A15C5)2phen)(CO)3Br] (1) with the aim to study the spectroscopic perturbations induced by the binding of the metal ions by this receptor. Interestingly, the rhenium complex 1 shows a particular behavior towards Hg(II) and Pb(II), as a result of the halide abstraction assisted by these metal ions. Additionally, in the presence of Cu(II) the interaction and coordination/binding with the macrocycle cavities is also presumed. The chemical reaction assisted by Hg(II) was confirmed by changes in the absorption, emission and FT–IR spectra of [Re(Cl2phen)(CO)3Br] (2) in presence of the heavy metal ion. These obtained profiles had similar patterns to the ones shown by the cationic complex [Re(Cl2phen)(CO)3(CH3CN)]PF6 (3). As final confirmation, this last complex was also studied in the presence of metal ions and its spectra remained unaltered.
揭示氮杂环三羰基铼 (I) 复合物在金属离子作用下的异常行为
我们合成了三种新的菲罗啉三羰基铼配合物,并对其进行了全面表征,以研究它们在金属离子存在下的光物理特性和光谱行为的变化。为此,在设计配合物时对菲罗啉和轴向配体都进行了修改。将 4,7-二氯-1,10-菲罗啉(Cl2phen)前体与两个单位的 1-氮杂-15-冠-5(1A15C5)进行官能化,得到了[Re((A15C5)2phen)(CO)3Br] 复合物(1),目的是研究金属离子与该受体结合时引起的光谱扰动。有趣的是,铼络合物 1 对 Hg(II) 和 Pb(II) 显示出特殊的行为,这是这些金属离子协助卤化物抽取的结果。此外,在 Cu(II)存在的情况下,还可以推测与大循环空腔发生了相互作用和配位/结合。重金属离子存在时,[Re(Cl2phen)(CO)3Br] (2) 的吸收、发射和傅立叶变换红外光谱的变化证实了 Hg(II) 的化学反应。这些光谱曲线与阳离子络合物 [Re(Cl2phen)(CO)3(CH3CN)]PF6 (3) 的光谱曲线相似。作为最后的确认,我们还在有金属离子存在的情况下研究了最后一种复合物,其光谱保持不变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


