Design and Biophysical Characterization of Second-Generation cyclic peptide LAG-3 inhibitors for cancer immunotherapy
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Lymphocyte activation gene 3 (LAG-3) is an inhibitory immune checkpoint crucial for suppressing the immune response against cancer. Blocking LAG-3 interactions enables T cells to recover their cytotoxic capabilities and diminishes the immunosuppressive effects of regulatory T cells. A cyclic peptide (Cys-Val-Pro-Met-Thr-Tyr-Arg-Ala-Cys, disulfide bridge: 1–9) was recently reported as a LAG-3 inhibitor. Based on this peptide, we designed 19 derivatives by substituting tyrosine residue to maximize LAG-3 inhibition. Screening via TR-FRET assay identified 8 outperforming derivatives, with cyclic peptides 12 [Tyr6(L-3-CN-Phe)], 13 [Tyr6(L-4-NH2-Phe)], and 17 [Tyr6(L-3,5-DiF-Phe)] as top candidates. Cyclic peptide 12 exhibited the highest inhibition (IC50 = 4.45 ± 1.36 µM). MST analysis showed cyclic peptides 12 and 13 bound LAG-3 with KD values of 2.66 ± 2.06 µM and 1.81 ± 1.42 µM, respectively, surpassing the original peptide (9.94 ± 4.13 µM). Docking simulations revealed that cyclic peptide 12 exhibited significantly enhanced binding, with a docking score of −7.236 kcal/mol, outperforming the original peptide (−5.236 kcal/mol) and cyclic peptide 5 (L-4-CN-Phe) (−5.131 kcal/mol). A per-residue decomposition of the interaction energy indicated that the 3-cyano group in cyclic peptide 12 contributes to a more favorable conformation, yielding an interaction energy of −9.22 kcal/mol with Phe443 of MHC-II, compared to −6.03 kcal/mol and −5.619 kcal/mol for cyclic peptides 0 and 5, respectively. Despite promising in vitro results, cyclic peptide 12 failed to inhibit tumor growth in vivo, underscoring the importance of dual immunotherapies targeting several immune checkpoints to achieve anti-tumor efficacy.
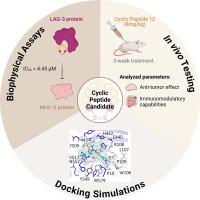
用于癌症免疫疗法的第二代环肽 LAG-3 抑制剂的设计与生物物理特性分析
淋巴细胞活化基因 3(LAG-3)是一种抑制性免疫检查点,对抑制抗癌免疫反应至关重要。阻断 LAG-3 的相互作用可使 T 细胞恢复其细胞毒性能力,并削弱调节性 T 细胞的免疫抑制作用。最近有报道称一种环肽(Cys-Val-Pro-Met-Thr-Tyr-Arg-Ala-Cys,二硫桥:1-9)可作为 LAG-3 抑制剂。在该肽的基础上,我们通过取代酪氨酸残基设计了 19 种衍生物,以最大限度地抑制 LAG-3。通过 TR-FRET 分析筛选出 8 种性能更优越的衍生物,其中环肽 12 [Tyr6(L-3-CN-Phe)]、13 [Tyr6(L-4-NH2-Phe)] 和 17 [Tyr6(L-3,5-DiF-Phe)] 为最佳候选。环肽 12 的抑制率最高(IC50 = 4.45 ± 1.36 µM)。MST 分析表明,环肽 12 和 13 与 LAG-3 结合的 KD 值分别为 2.66 ± 2.06 µM 和 1.81 ± 1.42 µM,超过了原始肽(9.94 ± 4.13 µM)。对接模拟显示,环肽 12 的结合力明显增强,对接得分为 -7.236 kcal/mol,优于原肽(-5.236 kcal/mol)和环肽 5(L-4-CN-Phe)(-5.131 kcal/mol)。按残基分解的相互作用能表明,环肽 12 中的 3-氰基有助于形成更有利的构象,与 MHC-II 的 Phe443 的相互作用能为 -9.22 kcal/mol,而环肽 0 和环肽 5 的相互作用能分别为 -6.03 kcal/mol 和 -5.619 kcal/mol。尽管体外实验结果很好,但环肽 12 未能抑制肿瘤在体内的生长,这凸显了针对多个免疫检查点的双重免疫疗法取得抗肿瘤疗效的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


