Synthesis and Understanding of the Role of Donor-Tetracyanobutadiene in Porphyrin β-Periphery toward Ultrafast Charge Transfer Dynamics
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
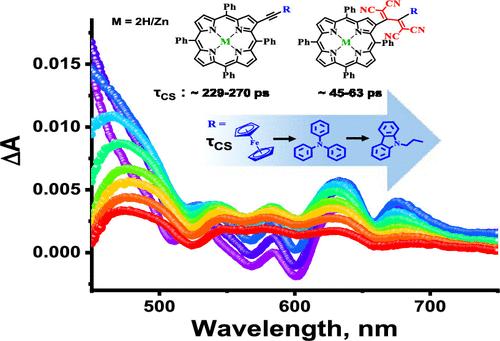
The porphyrins, structurally analogous to chlorophyll pigment, have drawn significant interest in mimicking natural photosynthetic processes in energy conversion applications. In this work, a new set of β-substituted free-base (H2P) and zinc-porphyrins (ZnP) have been designed (5–19) and synthesized employing ferrocene (Fc), triphenylamine (TPA), and carbazole (Cz) as secondary donors (D) and further incorporated tetracyanobuta-1,3-diene (TCBD) as strong electron acceptor (A) entity following Sonogashira cross-coupling and subsequent [2 + 2] cycloaddition–retroelectrocyclization reactions. Steady-state optical data exhibit a broad absorption in the 650–800 nm region, particularly in 15–19, corroborating ground-state charge polarization leading to intramolecular charge transfer (CT) in these systems. Strong fluorescence quenching in all of the systems (5–19) compared to the control compounds (C1 and C2) further suggests excited-state nonradiative photoprocesses predominate in these β-substituted dyads and triads, particularly after TCBD incorporations (15–19). Though the secondary donors quickly oxidize in 5–10, the same becomes difficult in 15–19, indicating an electronic influence of TCBD, leading to the respective formation of MP•–-D•+ and MP•+-A•–-D (MP = 2H or Zn) charge-separated (CS) species in a polar environment, which the molecular orbital positioning of the CT entities from computational studies has also justified. Finally, spectral and temporal dynamics of different photoproducts in these compounds have been assessed from femtosecond transient absorption studies, and subsequent fitting of the transient data identifies Cz contributing to the most stable and thus long-lived CS states, brightening their outstanding promise in solar energy harvesting and related electronic applications.
合成并了解卟啉 β-外围的供体-四氰丁二烯在超快电荷转移动力学中的作用
卟啉在结构上类似于叶绿素色素,在模仿自然光合作用过程的能量转换应用中引起了极大的兴趣。在这项工作中,利用二茂铁(Fc)、三苯胺(TPA)作为二级供体(D)和咔唑(Cz),设计(5-19)并合成了一组新的β-取代游离基(H2P)和锌-卟啉(ZnP)、二茂铁(Fc)、三苯胺(TPA)和咔唑(Cz)作为二级供体(D),并进一步加入四氰丁二烯(TCBD)作为强电子受体(A),经过 Sonogashira 交叉偶联和随后的[2 + 2]环加成-逆电环化反应合成了这些实体。稳态光学数据显示,在 650-800 nm 波段,尤其是在 15-19 波段,存在广泛的吸收,这证实了这些体系中导致分子内电荷转移(CT)的基态电荷极化。与对照化合物(C1 和 C2)相比,所有体系(5-19)都存在强烈的荧光淬灭,这进一步表明激发态非辐射光过程在这些 β 取代的二元和三元化合物中占主导地位,尤其是在加入 TCBD 之后(15-19)。虽然二级供体在 5-10 中会迅速氧化,但在 15-19 中却很难氧化,这表明 TCBD 的电子影响导致在极性环境中分别形成了 MP-D-+ 和 MP-+-A--D(MP = 2H 或 Zn)电荷分离(CS)物种,计算研究中 CT 实体的分子轨道定位也证明了这一点。最后,飞秒瞬态吸收研究评估了这些化合物中不同光产物的光谱和时间动态,随后的瞬态数据拟合确定了 Cz 对最稳定的 CS 状态做出了贡献,从而延长了 CS 状态的寿命,为它们在太阳能收集和相关电子应用领域的前景带来了光明。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


