Crystals of Organic Acid–Base Complexes Defy the ΔpKa Rule Under Compression
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Crystals of organic acid–base adducts are major components in active pharmaceutical ingredients. These 1:1 adducts either form a cocrystal with a hydrogen-bonded motif or a salt by transfer of a proton from the acid to the base. As a rule of thumb, if the difference in pKa between the protonated base and the acid (ΔpKa) is < −1, a cocrystal is expected, while ΔpKa > 4 leads to a salt, and in the intermediate zone (−1 ≤ ΔpKa ≤ 4) both cocrystal and salt are observed. The preferred crystalline form for 1:1 adducts of pyridine, pyridazine, pyrazine, and furan with formic acid is elucidated using genetic algorithm-assisted first-principles crystal structure predictions. In agreement with the ΔpKa rule, all the adducts stabilize as H-bonded cocrystals under ambient pressure. However, under isotropic pressure, formic acid transfers the protons to the three nitrogenous bases, forming salts of pyridinium formate, pyridazinium formate, and pyrazinium formate. External pressure is found to dictate the cocrystal–salt equilibrium. Critical pressure (Pc) required to induce cocrystal → salt conversion for formic acid··· pyridine/pyridazine/pyrazine is 3, 5, and 15 GPa, respectively. Compression is shown to enhance the electrostatic interactions between the molecules, leading to additional stabilization of the ionic configurations, namely, N+-H···O– in salts vis-à-vis the neutral N–H···O motifs in the cocrystals. Violating the ΔpKa rule, Pc overcomes the free energy required for the proton transfer (ΔGPT) to stabilize the salts. The very high ΔGPT = 177.9 kcal/mol for the furan···formic acid adduct prevents salt formation even at 30 GPa. Apart from thermodynamic and kinetic control during crystallization, pressure acts as a key control for organic acid–base adducts.
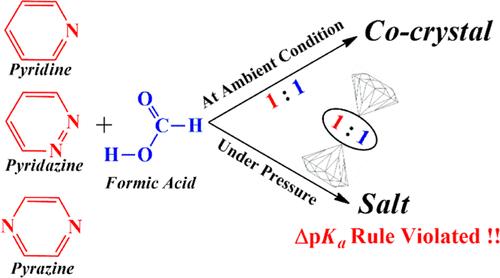
有机酸碱复合物晶体在压缩条件下违背 ΔpKa 规则
有机酸碱加合物晶体是活性药物成分中的主要成分。这些 1:1 加合物通过质子从酸转移到碱而形成具有氢键结构的共晶体或盐。根据经验,如果质子化的碱和酸之间的 pKa 差值(ΔpKa)为 <-1,则会形成共晶体,而 ΔpKa > 4 则会形成盐,在中间区域(-1 ≤ ΔpKa ≤ 4)会同时出现共晶体和盐。利用遗传算法辅助的第一原理晶体结构预测,阐明了吡啶、哒嗪、吡嗪和呋喃与甲酸的 1:1 加合物的优选结晶形式。与 ΔpKa 规则一致,所有加合物在常压下都稳定为 H 键共晶体。然而,在各向同性压力下,甲酸会将质子转移到三个含氮碱基上,形成甲酸吡啶鎓盐、甲酸哒嗪鎓盐和甲酸吡嗪鎓盐。外部压力决定了共晶-盐的平衡。诱导甲酸--吡啶/哒嗪/吡嗪的共晶→盐转化所需的临界压力(Pc)分别为 3、5 和 15 GPa。研究表明,压缩增强了分子间的静电相互作用,从而使离子构型更加稳定,即盐中的 N+-H-O- 与共晶体中的中性 N-H-O 结构相比更加稳定。与 ΔpKa 规则相反,Pc 克服了质子转移所需的自由能(ΔGPT),从而稳定了盐。呋喃--甲酸加合物的ΔGPT = 177.9 kcal/mol,即使在 30 GPa 下也能阻止盐的形成。除了结晶过程中的热力学和动力学控制之外,压力也是有机酸碱加合物的关键控制因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


